Abstract
Vaso-occlusion, hemolysis, and oxidative stress are hallmarks of sickle cell disease (SCD). This pathology is accompanied by systemic endothelial activation, rendering the endothelium more adhesive for blood cells, including sickle erythrocytes. Activated endothelial cells display or secrete several adhesive molecules, including von Willebrand factor (VWF). We assessed several VWF parameters in SCD patients at baseline: multimer pattern, antigen concentration (VWF:Ag), activation factor (VWF:AF), and total active VWF (VWF:TA). VWF:AF was determined using a llama nanobody (AU/VWFa-11) that detects a platelet-binding conformation of the A1 domain; VWF:TA was calculated by multiplying VWF:Ag by VWF:AF. SCD plasma contained elevated VWF:Ag and ultralarge VWF multimers. VWF:TA, a measure of total VWF reactivity, correlated closely with hemolysis, as determined by serum lactate dehydrogenase. ADAMTS13 activity and antigen were normal in all patients. These findings suggest an important role for hyperreactive VWF in SCD pathology and connect SCD to other microangiopathies, particularly thrombotic thrombocytopenic purpura.
Introduction
Sickle cell disease (SCD) results from inheritance of a mutant β-globin allele (Glu6Val),1 either as 2 copies or with an allele specifying deficient or defective β-globin, yielding rigid, adhesive, lysis-prone erythrocytes.2,3 These properties account for the clinical manifestations of SCD, including chronic hemolytic anemia and episodic vaso-occlusion, affecting many organs. Patients with the highest hemolytic rates are at risk for a syndrome composed of pulmonary artery hypertension, priapism, and leg ulcers.4
Several adhesive molecules have been implicated in sickle vaso-occlusion, including von Willebrand factor (VWF), a multimeric glycoprotein better known for mediating platelet adhesion.5 Acutely activated endothelial cells can release massive quantities of very large and hyperadhesive VWF molecules (ultra-large VWF [ULVWF]) capable of spontaneously binding platelets6,7 and erythrocytes, especially sickle cells.8-10 Normally, ULVWF is cleaved rapidly by the metalloproteinase ADAMTS13 into smaller, less adhesive multimers,11 preventing disastrous complications, such as thrombotic thrombocytopenic purpura.12 Elevated plasma VWF levels have been reported in SCD,13,14 but studies on the structure and reactivity of VWF in this disease are lacking.
Here we examine VWF quantity, reactivity, and multimer composition, and ADAMTS13 antigen and activity in a cohort of SCD patients at disease baseline (ie, not in acute crisis) and correlate these parameters with parameters of hemolysis, a marker of disease severity.
Methods
Patients
Collection of blood samples and clinical data from SCD patients at disease baseline was approved by the Institutional Review Board of the University of Washington. All analyses were carried out blinded to the identity and clinical characteristics of the patients.
VWF multimers
The VWF multimer pattern was evaluated by 1% agarose gel electrophoresis followed by Western blotting with a polyclonal VWF antibody, as described.15
VWF parameters
The VWF:Ag concentration was measured by enzyme-linked immunosorbent assay (ELISA).
Active VWF was detected by ELISA,16 using the llama nanobody AU/VWFa-11 (which detects a gain-of-function conformation of the A1 domain) as the capture antibody and polyclonal VWF antibody to detect bound VWF. The slope of VWF captured to VWF added for test plasmas was divided by the slope for pooled normal plasma, and this ratio was designated the VWF activation factor (VWF:AF).16
Total active VWF (VWF:TA) was determined by multiplying VWF antigen (VWF:Ag) by VWF:AF.
ADAMTS13 parameters
ADAMTS13 antigen was examined using an ELISA kit (American Diagnostica) per the manufacturer's instructions.
ADAMTS13 activity was determined using a peptide substrate as described previously,17 using pooled normal plasma as a standard.
Statistical analysis
The relationships between lactate dehydrogenase (LDH) and VWF parameters were assessed by fitting mixed-effects regression models.
Results and discussion
Patients
Of the 13 SCD patients examined, 9 were homozygous for HbS (HbSS), 2 had sickle-β+ thalassemia, one had sickle-β0 thalassemia, and one had HbSC. Patient characteristics and clinical laboratory values are shown in Table 1. Nine of the 13 had been prescribed hydroxyurea; 4 of 9 reported variable noncompliance. None was transfused within 30 days of blood sampling.
Sickle cell patient characteristics and laboratory values
| Patient no. . | Age, y/sex . | Genotype . | Hydroxyurea . | Leukocytes, 103/μL . | Platelets, 103/μL . | Reticulocytes, 106/μL . | Hematocrit, % . | LDH, units/L . | ADAMTS13,* % . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity . | Antigen . | Specific activity . | |||||||||
| 1 | 50/F | SS | Yes | 6.62 | 284 | 175 | 29 | 381 | — | — | — |
| 5.23 | 228 | 125 | 28 | 225 | 60 | 72 | 84 | ||||
| 10.52 | 326 | 82 | 25 | 342 | 94 | 78 | 120 | ||||
| 2 | 34/M | SS | Yes† | 17.94 | 576 | 205 | 25 | 465 | 63 | 115 | 55 |
| 17.40 | 653 | 170 | 25 | 491 | 137 | 119 | 115 | ||||
| 3 | 45/M | SS | Yes | 6.79 | 554 | 311 | 24 | 282 | |||
| 8.71 | 317 | 211 | 29 | 514 | 99 | 99 | 100 | ||||
| 4 | 49/M | SS | Yes | 5.48 | 150 | 244 | 25 | 394 | 112 | 116 | 97 |
| 5 | 25/M | SS | Yes† | 12.74 | 359 | 259 | 26 | 210 | 86 | 113 | 76 |
| 6 | 26/F | SS | No | 9.92 | 368 | 294 | 26 | 485 | 106 | 108 | 98 |
| 7 | 23/F | SS | No | 16.26 | 413 | 447 | 23 | 378 | 87 | 112 | 78 |
| 8 | 48/M | SS | Yes† | 11.21 | 323 | 271 | 23 | 418 | 100 | 132 | 76 |
| 9 | 24/F | SS | Yes | 7.16 | 366 | 313 | 28 | 250 | 94 | 103 | 91 |
| 10 | 29/M | Sβ0 | Yes | 9.44 | 310 | 270 | 31 | 395 | 104 | 108 | 96 |
| 11.00 | 464 | 280 | 34 | 437 | 144 | 119 | 120 | ||||
| 11 | 24/M | SC | Yes† | 6.58 | 426 | 124 | 33 | 215 | 198 | 139 | 142 |
| 6.95 | 388 | 111 | 30 | 210 | 159 | 124 | 128 | ||||
| 12 | 19/F | Sβ+ | No | 11.66 | 443 | 326 | 22 | 235 | 113 | 119 | 95 |
| 13 | 21/F | Sβ+ | No | 4.84 | 401 | 192 | 33 | 220 | 110 | 128 | 86 |
| Normal range | 4.3-10 | 150-400 | 20-65 | 36-45 | 80-190 | 100 | 100 | 100 | |||
| Patient no. . | Age, y/sex . | Genotype . | Hydroxyurea . | Leukocytes, 103/μL . | Platelets, 103/μL . | Reticulocytes, 106/μL . | Hematocrit, % . | LDH, units/L . | ADAMTS13,* % . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity . | Antigen . | Specific activity . | |||||||||
| 1 | 50/F | SS | Yes | 6.62 | 284 | 175 | 29 | 381 | — | — | — |
| 5.23 | 228 | 125 | 28 | 225 | 60 | 72 | 84 | ||||
| 10.52 | 326 | 82 | 25 | 342 | 94 | 78 | 120 | ||||
| 2 | 34/M | SS | Yes† | 17.94 | 576 | 205 | 25 | 465 | 63 | 115 | 55 |
| 17.40 | 653 | 170 | 25 | 491 | 137 | 119 | 115 | ||||
| 3 | 45/M | SS | Yes | 6.79 | 554 | 311 | 24 | 282 | |||
| 8.71 | 317 | 211 | 29 | 514 | 99 | 99 | 100 | ||||
| 4 | 49/M | SS | Yes | 5.48 | 150 | 244 | 25 | 394 | 112 | 116 | 97 |
| 5 | 25/M | SS | Yes† | 12.74 | 359 | 259 | 26 | 210 | 86 | 113 | 76 |
| 6 | 26/F | SS | No | 9.92 | 368 | 294 | 26 | 485 | 106 | 108 | 98 |
| 7 | 23/F | SS | No | 16.26 | 413 | 447 | 23 | 378 | 87 | 112 | 78 |
| 8 | 48/M | SS | Yes† | 11.21 | 323 | 271 | 23 | 418 | 100 | 132 | 76 |
| 9 | 24/F | SS | Yes | 7.16 | 366 | 313 | 28 | 250 | 94 | 103 | 91 |
| 10 | 29/M | Sβ0 | Yes | 9.44 | 310 | 270 | 31 | 395 | 104 | 108 | 96 |
| 11.00 | 464 | 280 | 34 | 437 | 144 | 119 | 120 | ||||
| 11 | 24/M | SC | Yes† | 6.58 | 426 | 124 | 33 | 215 | 198 | 139 | 142 |
| 6.95 | 388 | 111 | 30 | 210 | 159 | 124 | 128 | ||||
| 12 | 19/F | Sβ+ | No | 11.66 | 443 | 326 | 22 | 235 | 113 | 119 | 95 |
| 13 | 21/F | Sβ+ | No | 4.84 | 401 | 192 | 33 | 220 | 110 | 128 | 86 |
| Normal range | 4.3-10 | 150-400 | 20-65 | 36-45 | 80-190 | 100 | 100 | 100 | |||
Patients 1,2,3,10, and 11 were tested more than once.
LDH indicates lactate dehydrogenase in plasma; SS, homozygous sickle disease; SC, hemoglobin SC disease; Sβ+, sickle β (+) thalassemia; and Sβ0, sickle β (0) thalassemia.
Values were expressed relative to pooled normal plasma.
Patient reported intermittent noncompliance.
VWF analysis
We evaluated VWF multimer composition, VWF:Ag, VWF:AF, and VWF:TA.
ULVWF in SCD plasma.
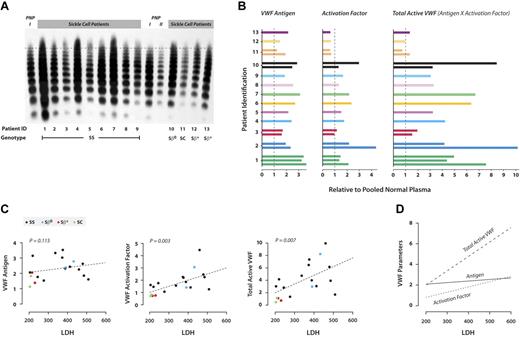
By agarose gel electrophoresis, many of the SCD plasmas had higher VWF levels (higher band intensities) and larger multimers than did normal plasma (Figure 1A).
VWF reactivity in SCD. (A) VWF multimer distribution. The VWF multimer distribution was examined in plasma from either sickle cell patients or pooled normal plasma by electrophoresis followed by Western blotting. For each of the sickle cell patients, 0.5 μL of plasma was loaded. For pooled normal plasma, PNP I and PNP II, 1 μL and 0.5 μL of plasma was loaded, respectively. The samples were labeled according to the patient identification number and grouped by genotype. The dashed line is drawn slightly above the highest multimer in pooled normal plasma. The multimers above the dashed line in samples of sickle cell patients are considered ULVWF. (B) VWF parameters. Data from different patients are presented in different colors; patients 1, 2, 3, 10, and 11 provided multiple samples. The values expressed are relative to those obtained with pooled normal plasma, which was arbitrarily assigned a value of 1, as indicated by the dashed lines in each panel. The assays were performed independently 2 or 3 times per sample; average values are shown. VWF antigen was measured by sandwich ELISA using a polyclonal VWF antibody as the capture antibody, and the captured VWF was detected with a horseradish peroxidase–conjugated polyclonal VWF antibody. VWF:AF was determined using AU/VWFa-11 as the capture antibody in ELISA, and the bound VWF was detected by a horseradish peroxidase–conjugated polyclonal VWF antibody. VWF:TA in the plasma was determined by multiplying the VWF antigen concentration by the VWF-AF. (C) Correlation between VWF parameters and LDH. The association between LDH and each of the VWF variables was assessed by fitting mixed-effects regression models. The estimated regression line has been superimposed on the corresponding graph. The number of patients was 13, and the number of observations was 19. LDH concentration correlated significantly with VWF:AF (P = .003) and VWF:TA (P = .007) but not with VWF antigen concentration (P = .115). Patients with different genotypes are identified by different colors: black represents SS; blue, Sβ0; red, Sβ+; and green, SC. (D) VWF:TA correlates best with LDH. When the regression lines for VWF antigen, VWF:AF, and VWF:TA were plotted on the same scale, the slope of the regression line between VWF:TA and LDH was the steepest, indicating that VWF:TA is more sensitive to changes in LDH than VWF:Ag or VWF:AF.
VWF reactivity in SCD. (A) VWF multimer distribution. The VWF multimer distribution was examined in plasma from either sickle cell patients or pooled normal plasma by electrophoresis followed by Western blotting. For each of the sickle cell patients, 0.5 μL of plasma was loaded. For pooled normal plasma, PNP I and PNP II, 1 μL and 0.5 μL of plasma was loaded, respectively. The samples were labeled according to the patient identification number and grouped by genotype. The dashed line is drawn slightly above the highest multimer in pooled normal plasma. The multimers above the dashed line in samples of sickle cell patients are considered ULVWF. (B) VWF parameters. Data from different patients are presented in different colors; patients 1, 2, 3, 10, and 11 provided multiple samples. The values expressed are relative to those obtained with pooled normal plasma, which was arbitrarily assigned a value of 1, as indicated by the dashed lines in each panel. The assays were performed independently 2 or 3 times per sample; average values are shown. VWF antigen was measured by sandwich ELISA using a polyclonal VWF antibody as the capture antibody, and the captured VWF was detected with a horseradish peroxidase–conjugated polyclonal VWF antibody. VWF:AF was determined using AU/VWFa-11 as the capture antibody in ELISA, and the bound VWF was detected by a horseradish peroxidase–conjugated polyclonal VWF antibody. VWF:TA in the plasma was determined by multiplying the VWF antigen concentration by the VWF-AF. (C) Correlation between VWF parameters and LDH. The association between LDH and each of the VWF variables was assessed by fitting mixed-effects regression models. The estimated regression line has been superimposed on the corresponding graph. The number of patients was 13, and the number of observations was 19. LDH concentration correlated significantly with VWF:AF (P = .003) and VWF:TA (P = .007) but not with VWF antigen concentration (P = .115). Patients with different genotypes are identified by different colors: black represents SS; blue, Sβ0; red, Sβ+; and green, SC. (D) VWF:TA correlates best with LDH. When the regression lines for VWF antigen, VWF:AF, and VWF:TA were plotted on the same scale, the slope of the regression line between VWF:TA and LDH was the steepest, indicating that VWF:TA is more sensitive to changes in LDH than VWF:Ag or VWF:AF.
VWF:Ag was elevated in almost all of the patients (Figure 1B), from 1.2- to 3.6-fold the concentration in normal plasma.
VWF activity in SCD.
VWF-TA.
The thrombotic potential of VWF is likely to correlate with both its concentration and its adhesiveness as measured by the VWF:AF. VWF:TA simultaneously accounts for both VWF quantity and adhesiveness and is defined as the product of VWF:Ag and VWF:AF. The VWF:TA represents the value for patient plasma relative to normal plasma. In our patients, VWF:TA ranged from 0.7 to 10.1 (Figure 1B), with 11 of the 13 patients having increased VWF:TA.
Correlation of VWF parameters with biologic parameters
We correlated VWF:Ag, VWF:AF, and VWF:TA from the SCD patients with the parameters listed in Table 1 and in the following 3 paragraphs.
VWF:AF and VWF:TA correlate with plasma LDH.
VWF parameters and other clinical values.
None of the VWF parameters correlated with any of several other clinical parameters, including hemoglobin/hematocrit, mean corpuscular volume, bilirubin, ferritin, creatinine, or numbers of leukocytes, platelets, or reticulocytes.
VWF parameters of the SCD patients with different genotypes.
VWF parameters in patients with genotypes associated with less severe disease (Sβ+ and SC) were similar to those of pooled normal plasma (Figure 1C). On the other hand, VWF parameters in the Sβ0 patient were more similar to those of the SS patients, which were generally much higher than normal plasma. Similarly, the SC and Sβ+ displayed normal multimer patterns (Figure 1A), whereas the Sβ0 patient contained increased quantities of very large multimers. VWF parameters in the SS patients were highly variable, consistent with a wide spectrum of disease severity.
ADAMTS13 antigen and activity
Neither ADAMTS13 antigen nor activity was significantly deficient in any of the SCD patients (Table 1). ADAMTS13-specific activity (activity/concentration) was slightly decreased in 6 patients.
This is the first study of VWF conformation and reactivity in SCD. Multimer analysis indicated that many of the SCD patients had elevated quantities of both total VWF and large multimers. This was corroborated by the elevated antigen levels and activation factors, integrated in the parameter VWF:TA, which takes into account both the abundance and reactivity of circulating VWF. VWF:TA also assesses the adhesiveness of smaller VWF multimers, which are not reactive in normal plasma but may be hyperadhesive in diseases such as thrombotic thrombocytopenic purpura.19 That elevated nanobody binding reflects increased VWF adhesive activity in SCD plasma was shown by the fact that, at equivalent VWF concentrations, SCD plasma was much more potent at agglutinating platelets than was normal plasma (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Other studies failed to demonstrate a correlation between VWF concentration and disease severity in SCD,14,20 a finding we verified. On the other hand, in this study, VWF:TA correlated very strongly with hemolysis, suggesting that hemolysis in SCD is microangiopathic, with lysis-prone sickle erythrocytes suffering damage by having to navigate through a mesh of VWF strands. This notion is supported by the description of a thrombotic thrombocytopenic purpura–like syndrome in SCD patients.21,22 The binding of sickle erythrocytes to endothelial cell–immobilized VWF could also increase hemolysis by slowing erythrocyte transit enough to induce hemoglobin deoxygenation and polymerization, consistent with the finding that sickle erythrocyte adhesion to ULVWF was highest at a fluid shear stress of 0.5 dyne/cm2, approximately that of postcapillary venules.23
Hyperreactive VWF probably accumulates in SCD by a combination of increased secretion, defective clearance, and impaired processing by ADAMTS13. Impaired processing is not the result of ADAMTS13 deficiency but could reflect resistance of VWF to proteolysis, possibly because of oxidation of Met1606 at the ADAMTS13 cleavage site.24 Because a portion of ULVWF remains tethered to the endothelial surface after endothelial activation,6 increased circulating ULVWF in SCD probably reflects a similar increase in endothelium-associated ULVWF.
In conclusion, we provide evidence that SCD at baseline is associated with high concentrations of hyperadhesive VWF, which correlates closely with the rate of hemolysis. Whether VWF quantity and reactivity increase even further during acute exacerbations will be particularly interesting to examine. Thus, VWF could represent a therapeutic target to ameliorate hemolysis in SCD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the National Heart, Lung, and Blood Institute (R01-HL091153; J.A.L., J.C.).
National Institutes of Health
Authorship
Contribution: J.C. designed and performed experiments, analyzed data, and cowrote the manuscript; W.E.H. collected the patient samples, analyzed data, and cowrote the manuscript; J.L. performed experiments; P.J.L. and P.G.d.G. provided us with vital reagents for experiments, contributed important intellectual insights into data interpretation, and edited the manuscript; and J.A.L. directed the project, designed and interpreted experiments, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: José A. López, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104, e-mail: josel@psbcresearch.org.
References
Author notes
J.C. and W.E.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal