Abstract
Adult T-cell leukemia-lymphoma (ATL) is an aggressive disease, incurable by standard chemotherapy. NK314, a new anticancer agent possessing inhibitory activity specific for topoisomerase IIα (Top2α), inhibited the growth of various ATL cell lines (50% inhibitory concentration: 23-70nM) with more potent activity than that of etoposide. In addition to the induction of DNA double-strand breaks by inhibition of Top2α, NK314 induced degradation of the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs), resulting in impaired DNA double-strand break repair. The contribution of DNA-PK to inhibition of cell growth was affirmed by the following results: NK314 inhibited cell growth of M059J (a DNA-PKcs–deficient cell line) and M059K (a cell line with DNA-PKcs present) with the same potency, whereas etoposide exhibited weak inhibition of cell growth with M059K cells. A DNA-PK specific inhibitor, NU7026, enhanced inhibitory activity of etoposide on M059K as well as on ATL cells. These results suggest that NK314 is a dual inhibitor of Top2α and DNA-PK. Because ATL cells express a high amount of DNA-PKcs, NK314 as a dual molecular targeting anticancer agent is a potential therapeutic tool for treatment of ATL.
Introduction
Adult T-cell leukemia-lymphoma (ATL) is an aggressive and incurable disease caused by type 1 human T-lymphotropic virus (HTLV-1).1-3 HTLV-1 is a retrovirus endemic to southern Japan and several other countries. After infection, polyclonal expansion of HTLV-1–infected T cells progresses to a state of monoclonal proliferation, with subsequent occurrence of overt ATL after several decades.2,3 There is presently no cure for ATL; median survival time is only 13 months in advanced ATL, such as acute and lymphoma types.4,5 Various clinical trials using new therapeutic strategies have been conducted toward improving survival time of patients with aggressive ATL. Among the protocols using combination chemotherapy, VCAP-AMP-VECP therapy consisting of a dose-intensified multiagent chemotherapy improved overall survival with ATL.4 The protocol included agents overcoming multidrug resistant-related genes, which are frequently expressed in ATL cells.6,7 Allogeneic hematopoietic stem cell transplantation for young ATL patients is a promising approach, showing an estimated overall 3-year survival of 45%, but the study size was inadequate.8,9 Treatment with a large dose of zidovudine and interferon-α also produced a good response in patients with acute-type ATL.10,11
Recent analyses of molecular mechanisms associated with the occurrence of ATL revealed several molecular targets responsible for cellular transformation and disease progression that could be effective targets for new therapeutic approaches. Based on these studies, targeting therapies for various molecules, such as Tax, NF-κB, Akt/PKB, mTOR, CD25, CD52, and chemokine receptor-4, have been developed under clinical or preclinical investigations using small molecules or monoclonal antibodies.12-23 The DNA repair system is also a promising target for sensitization of cancer treatment.24-26 Although genetic instability of proliferating cells is necessary for most cell types to become malignant, cells that are overly unstable genetically will die. Therefore, a treatment that blocks a particular DNA repair system can induce apoptotic cell death of cancer cells but not normal cells. In response to DNA double-strand breaks (DSBs), DNA repair can be mediated by 2 major pathways: homologous recombination and nonhomologous end-joining; more than 90% of DSBs in mammalian cells are repaired by nonhomologous end-joining.27 Nonhomologous end-joining in mammalian cells is achieved by core protein components, including Ku70/Ku80, DNA-dependent protein kinase (DNA-PK), XRCC4, DNA ligase IV, Artemis, and Cernunnos-XLF.28,29
NK314, a synthetic derivative of benzo[c]phenanthridine, is a unique anticancer agent possessing specific and potent inhibitory activity against topoisomerase IIα (Top2α).30-32 We found that NK314 inhibited ATL cell lines with greater potency than etoposide, a Top2 inhibitor frequently used in practice. Because the range of 50% inhibitory concentration values of NK314 for ATL cell lines and non-ATL cell lines differs from those of etoposide, we investigated other targets of NK314 for induction of apoptosis and inhibition of cell growth of ATL cell lines. In this paper, we demonstrate that NK314 induced degradation of DNA-PKcs and inhibited DNA DSBs repair. The results suggest that NK314 is a new type of anticancer drug possessing inhibitory activity against Top2α and DNA-PK, and so is a promising candidate for treatment of ATL.
Methods
Chemicals and antibodies
NK314 (Figure 1A) was synthesized at Nippon Kayaku and dissolved in distilled water before use.30-32 Etoposide was also synthesized at Nippon Kayaku. NU7026 was purchased from Sigma-Aldrich. Sources of antibodies were as follows: PARP-1, Ku70, and actin (Santa Cruz Biotechnology) and DNA-PKcs and Ku86 (Lab Vision Corporation).
Cell lines and culture conditions
The study protocol was approved by the Clinical Research Ethics Committee of Saga University; and when applicable, informed consent was obtained in compliance with the Declaration of Helsinki. Eight ATL cell lines were used: 6 ATL-derived cell lines and 2 HTLV-1–infected T-cell lines. ATN-1, KK1, ST1, and KOB were established from ATL patients and kindly provided by Dr Yasuaki Yamada of Nagasaki University Graduate School of Biomedical Sciences; the patient origins of these cells were confirmed by concordance of the integration sites of HTLV-1 proviral DNA and/or rearrangement profiles of the T-cell receptor β-chain gene.33-35 MT-1 was derived form a patient with ATL.36 MT-2 was established by cocultivation of human cord blood lymphocytes from a normal subject with peripheral blood mononuclear cells (PBMCs) from an ATL patient.37 OMT is an HTLV-1–infected T-cell line.38 MT-2 was kindly provided by Dr Kunitada Shimotono of Kyoto University, Japan. MOLT-4, Jurkat, M059J, and M059K cells were purchased from ATCC. Stable transformants with DNA-PK cDNA (MJ L24) or vector alone (MJ NA) introduced into M059J cells were established as described previously.39 MT-1, MT-2, ATN-1, MOLT-4, and Jurkat cells were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS). M059K, M059J, MJ L24, and MJ NA glioblastoma cell lines were cultured in F-12/Dulbecco modified Eagle medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% nonessential amino acids. OMT, KOB, KK1, and ST1 were interleukin-2 (IL-2) dependent and were cultured in RPMI 1640 containing 10% FBS with 10 ng/mL recombinant human IL-2, kindly provided by Takeda Chemical Industries. Normal PBMCs were collected from 5 healthy volunteers and cultured in RPMI 1640 containing 10% FBS.
Analysis of cell proliferation, cell cycle, and apoptosis
ATL, MOLT-4, and Jurkat cells (2 × 106 cells) were cultured in a 10-mL culture bottle for 1 hour and treated with the indicated concentrations of NK314 and etoposide, with or without the DNA-PK inhibitor NU7026 for 48 hours. The number of cells was determined with trypan blue staining or Cell Counting Kit-8 (Dojindo Molecular Technologies). Relative ratio of cell growth, calculated by dividing by the number of untreated cells, is shown as mean plus or minus SD of triplicate analyses. Cell-cycle analysis of ATL cells was performed using flow cytometry. Cells (1 × 106 cells) were treated with the indicated concentrations of NK314. Twenty-four hours after treatment, cells were collected and fixed with 2% paraformaldehyde followed by 70% ethanol. Cells were treated with RNase A (0.25 mg/mL) at 37°C for 30 minutes and stained with 50 μg/mL of propidium iodide (BD Biosciences). Cellular DNA content was analyzed using flow cytometry, and cell-cycle profiles were determined with CellQuest software Version 3.1 (BD Biosciences). Analysis of apoptosis was conducted using a MEBCYTO Apoptosis Kit (Medical & Biologic Laboratories). Cells treated with NK314 for 24 hours were simultaneously stained with annexin V–fluorescein isothiocyanate and propidium iodide according to the manufacturer's instructions. Stained cells were subjected to fluorescence-activated cell sorter analysis to determine the number of apoptotic cells. Induction of apoptosis was also assessed by degradation of PARP-1. Cell extracts were obtained after treatment with NK314 or etoposide at each time point, and the degradation of PARP-1 was determined by Western blot analysis.
Western blot analysis
Whole cell lysates of ATN-1,MT-1, and OMT cells were prepared from cells using lysis buffer containing 50mM Tris-HCl at pH 8.0, 150mM NaCl, 5mM MgCl2, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 40mM sodium fluoride, 1mM sodium orthovanadate, 1 μg/mL leupeptin, 10 μg/mL aprotinin, and 1mM phenylmethylsulfonyl fluoride, as reported previously.40 Protein was separated using a 10% NuPAGE electrophoresis system (Novex), transferred to a nitrocellulose membrane (Schleicher & Schuell), blocked with 5% milk at 4°C overnight, and finally reacted with primary antibodies. An enhanced chemiluminescence kit (GE Healthcare) was used for detection.
γH2AX measurement using flow cytometry, and irradiation
After treatment with the indicated concentration of NK314 for 1, 6, 24, or 48 hours, cells were harvested and washed with phosphate-buffered saline 3 times. γH2AX was detected using an H2AX Phosphorylation Assay Kit (Millipore). Cells were treated with RNase A (10 μg/mL) at 37°C for 30 minutes and counterstained with propidium iodide (25 μg/mL). Stained samples were analyzed using flow cytometry (BD Biosciences).
DNA repair capacity of NK314 after irradiation was evaluated as follows. After treatment with NK314 for 1 hour, cells were irradiated at 4 Gy, followed by detection of γH2AX using flow cytometry at the indicated period. Cells were irradiated using an IBL437C-I 137Cs γ-irradiator (CIS Bio International) at 3.5 Gy/min. DNA repair capacity was presented as time courses of γH2AX induction and relative ratios of γH2AX+ cells 5 minutes and 2 hours after γ-irradiation combined with compounds compared with γ-irradiation alone.
Colony-forming assay
Colony-forming assay was performed using MethoCult Express (Stem Cell Technologies). Whole bone marrow cells from a healthy volunteer were diluted with Iscove modified Dulbecco medium (Invitrogen) plus 2% FBS (Nichirei Corporation), and added to MethoCult Express. The cells were dispensed into 35-mL culture dishes at a final concentration of 1.5 × 104 cells/mL/dish. After culture at 37°C in 5% CO2 with more than or equal to 95% humidity for 7 days, all colonies containing more than 20 cells were counted.
Animal experiments
Animal experiments were approved by the Animal Research Ethics Committee of Saga University. MT-1 cells (5 × 105) were injected into the dorsal flanks of SCID mice (C.B-17/IcrCrj-scid/scid, Charles River Laboratories) after 4 Gy irradiation. A total of 10 or 20 mg/kg of NK314 or phosphate-buffered saline was injected intraperitoneally at days 14, 28, and 42 after inoculation. Tumor volumes, defined as (short axis)2 × (long axis)/2, were measured every 3 days and are expressed as mean plus or minus SD. Cell count in peripheral blood was obtained with a K-4500 auto blood cell counter (Sysmex Corporation). Biochemical analysis was performed by SRL.
Statistical analysis
Tumor volumes were compared using a 2-sided t test. Statistical analyses were conducted using SPSS Version 11.0J (SPSS Japan).
Results
NK314 inhibited cell growth and induced apoptosis via G2/M arrest of ATL-related cell lines
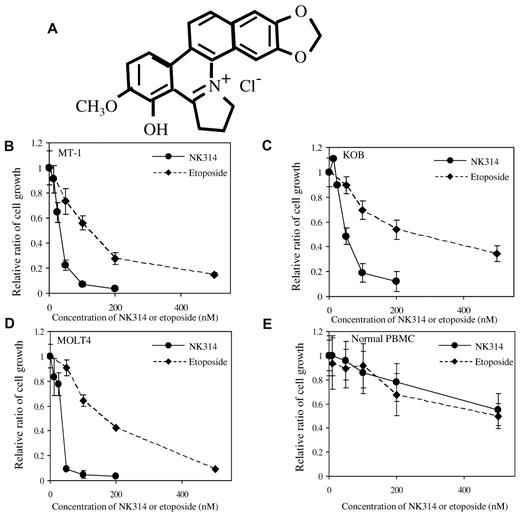
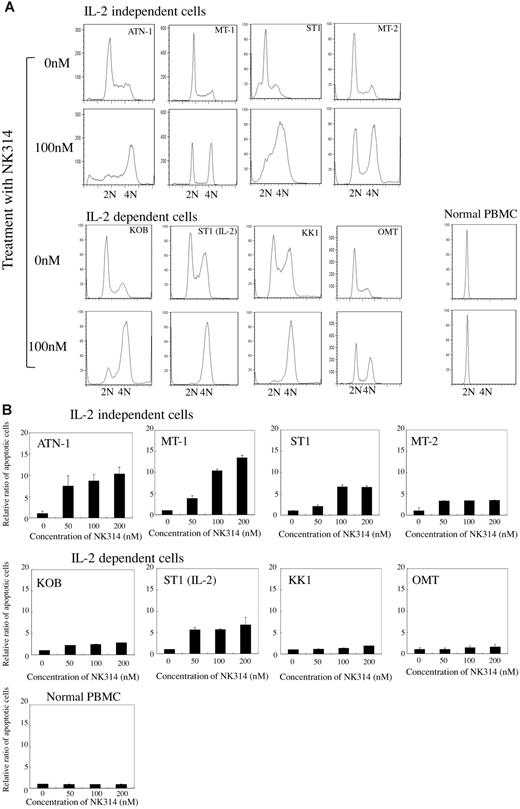
NK314 is a synthetic benzo[c]phenanthridine alkaloid that shows strong inhibitory activity against Top2α and antitumor activity.30-32 Because NK314 inhibits Top2α more specifically and more potently than etoposide, which is a key drug for ATL treatment, we investigated the antitumor activity of NK314 in ATL cells. Various ATL-related (6 ATL-derived cell lines and 2 HTLV-1–infected) T-cell lines were cultured with or without IL-2. Non-ATL T-cell leukemia cell lines, MOLT4, and Jurkat cells were also used in this study. NK314 or etoposide was added at various concentrations in culture media, and cell growth rates were determined with trypan blue staining 48 hours after treatment. NK314 inhibited cell growth of ATL cell lines in a dose-dependent manner. Representative results using an IL-2–independent cell line, MT-1, and an IL-2–dependent cell line, KOB, are shown in Figure 1B-C. The 50% inhibitory concentration values of NK314 and etoposide ranged from 22.6 to 71.3nM and 76.7 to 344.7nM, respectively (Table 1). Non-ATL cell lines, such as MOLT4 and Jurkat cells, were also more sensitive to NK314 than to etoposide. Inhibitory effects of cell growth by these compounds were weak on normal PBMCs. To examine the mechanisms of growth inhibition by NK314, we next investigated the effects of NK314 on the cell cycle and on induction of apoptosis in ATL cell lines and PBMCs. NK314 induced G2/M arrest dose-dependently on all cell lines examined, except PBMCs, which were kept in G0/G1 phase (Figure 2A). Etoposide also induced G2/M arrest on all cell lines examined (data not shown). Induction of G2/M arrest was associated with apoptosis analyzed by induction of annexin V and cleavage of PARP-1, although the activities of NK314 for induction of apoptosis on ATL cell lines varied (Figure 2B; supplemental Data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Among the cell lines, IL-2–independent cell lines, such as ATN-1 and MT-1, showed stronger induction of apoptosis compared with IL-2–dependent cell lines after treatment with NK314. Because various Top2 inhibitors have been reported to be inducers of G2/M arrest in cancer cell lines,41 it is possible that Top2 inhibition was achieved in the ATL cell lines, including IL-2–dependent cell lines, but that the activation of apoptotic pathway was relatively weak in these cell lines. NK314 did not evidence induction of annexin V or cleavage of PARP-1 on PBMCs.
NK314 inhibits cell growth of ATL cell lines. (A) Structure of NK314. (B-E) Inhibition of cell growth of ATL-related cell lines MT-1 (B), KOB (C), MOLT4 (D), and normal PBMCs (E) by treatment with NK314 or etoposide. NK314 (●) or etoposide (♦) were added at various concentrations in culture media, and cell growth rates were determined by trypan blue staining 48 hours after treatment. The relative ratio of cell growth is shown; values are mean ± SD of triplicate analyses.
NK314 inhibits cell growth of ATL cell lines. (A) Structure of NK314. (B-E) Inhibition of cell growth of ATL-related cell lines MT-1 (B), KOB (C), MOLT4 (D), and normal PBMCs (E) by treatment with NK314 or etoposide. NK314 (●) or etoposide (♦) were added at various concentrations in culture media, and cell growth rates were determined by trypan blue staining 48 hours after treatment. The relative ratio of cell growth is shown; values are mean ± SD of triplicate analyses.
Growth inhibition of ATL cell lines induced by NK314 or etoposide
| Cell line . | IC50, nM . | |
|---|---|---|
| NK314 . | Etoposide . | |
| IL-2–independent | ||
| ATN-1 | 38.3 | 135.8 |
| MT-1 | 34.8 | 115.0 |
| ST1 | 45.2 | 138.0 |
| MT-2 | 22.6 | 76.7 |
| IL-2–dependent | ||
| KOB | 58.2 | 248.6 |
| ST1 (IL-2) | 71.3 | 214.5 |
| KK1 | 60.1 | 194.2 |
| OMT | 51.3 | 344.7 |
| MOLT4 | 32.6 | 157.9 |
| Jurkat | 29.2 | 145.8 |
| Normal PBMCs | ≥ 500 | ≥ 500 |
| Cell line . | IC50, nM . | |
|---|---|---|
| NK314 . | Etoposide . | |
| IL-2–independent | ||
| ATN-1 | 38.3 | 135.8 |
| MT-1 | 34.8 | 115.0 |
| ST1 | 45.2 | 138.0 |
| MT-2 | 22.6 | 76.7 |
| IL-2–dependent | ||
| KOB | 58.2 | 248.6 |
| ST1 (IL-2) | 71.3 | 214.5 |
| KK1 | 60.1 | 194.2 |
| OMT | 51.3 | 344.7 |
| MOLT4 | 32.6 | 157.9 |
| Jurkat | 29.2 | 145.8 |
| Normal PBMCs | ≥ 500 | ≥ 500 |
Effect of NK314 on cell cycle and apoptosis in ATL cell lines. (A) Cell-cycle analysis using flow cytometry after NK314 treatment of ATL cells. ATL cells were treated with the indicated concentrations of NK314 and then fixed and stained with propidium iodide 24 hours after treatment. Cellular DNA content and cell cycle profiles were determined with CellQuest software Version 3.1 (BD Biosciences). (B) Annexin V and propidium iodide analysis of ATL cells treated with NK314. Cells treated with NK-314 for 24 hours were simultaneously stained with annexin V and propidium iodide according to the manufacturer's instructions. Stained cells were conducted to a fluorescence-activated cell sorter analysis to determine the number of apoptotic cells. The ratio of apoptotic cells was calculated as number of apoptotic cells treated with NK314 of the indicated concentrations divided by the number of untreated cells and expressed as mean ± SD of triplicate analyses.
Effect of NK314 on cell cycle and apoptosis in ATL cell lines. (A) Cell-cycle analysis using flow cytometry after NK314 treatment of ATL cells. ATL cells were treated with the indicated concentrations of NK314 and then fixed and stained with propidium iodide 24 hours after treatment. Cellular DNA content and cell cycle profiles were determined with CellQuest software Version 3.1 (BD Biosciences). (B) Annexin V and propidium iodide analysis of ATL cells treated with NK314. Cells treated with NK-314 for 24 hours were simultaneously stained with annexin V and propidium iodide according to the manufacturer's instructions. Stained cells were conducted to a fluorescence-activated cell sorter analysis to determine the number of apoptotic cells. The ratio of apoptotic cells was calculated as number of apoptotic cells treated with NK314 of the indicated concentrations divided by the number of untreated cells and expressed as mean ± SD of triplicate analyses.
NK314 inhibited repair of DNA DSBs induced by ionizing radiation in ATL cell lines
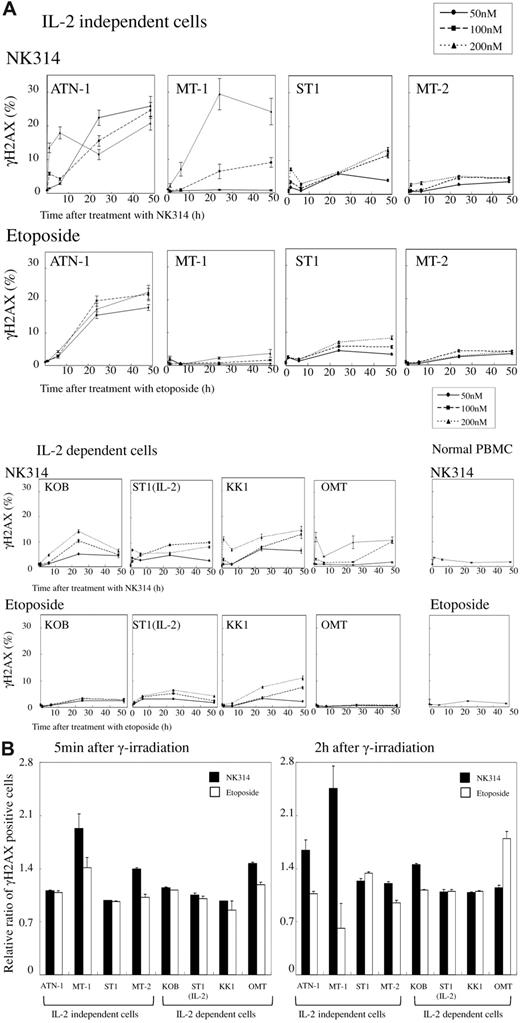
It has been reported that the inhibition of Top2α induces DNA DSBs, and recent papers suggest that inhibition of DNA damage response can overcome the drug resistance seen in cancer chemotherapy.24-26 We investigated the effects of NK314 on DNA damage response with or without irradiation. ATL cell lines and PBMCs were treated with NK314 with or without 4 Gy irradiation 1 hour after seeding. The induction of γH2AX was monitored by flow cytometry as a marker of DNA DSBs. In MT-1 cells, NK314 alone gradually increased the number of γH2AX+ cells 24 hours after treatment with apparently stronger potency than that of etoposide, and other IL-2–independent cell lines showed similar tendencies, except MT-2 cells (Figure 3A). However, induction of γH2AX+ cells by NK314 was weak in IL-2–dependent cell lines. These results were comparable with those of apoptosis. Among 8 cell lines examined, induction of DNA damage was more apparent and prolonged by treatment with NK314 compared with etoposide in 7 cell lines. Again, neither NK314 nor etoposide evidenced DNA damage in normal PBMCs. Combining NK314 or etoposide with 4 Gy irradiation induced remarkable induction of γH2AX+ cells 5 minutes after irradiation (supplemental Data). However, disappearance (indicating DNA damage repair) of the positive cells was apparently inhibited after treatment with NK314 in 4 of 8 cell lines examined (supplemental Data). We assessed the effects of these compounds on induction of DNA damage (γH2AX+ cells at 5 minutes) and impairment of DNA repair (γH2AX+ cells at 2 hours; Figure 3B). The induction pattern of γH2AX+ foci 5 minutes after irradiation was comparatively higher in cells treated with NK314, and the population of γH2AX+ foci was apparently larger with NK314 treatment compared with etoposide, especially in IL-2–independent cell lines. These results suggest that NK314 contributes to impairment of DNA repair as well as induction of DNA damage.
Induction of DNA DSB in ATL cells by NK314 or etoposide and comparison of DNA repair capacity in NK314- or etoposide-treated cells after irradiation. (A) After treatment with the indicated concentrations of NK-314 or etoposide, induction of γH2AX+ foci was determined by flow cytometry using an H2A.X Phosphorylation Assay Kit. The percentage of γH2AX+ foci was calculated by dividing by the number of cells without treatment by the compounds. (B) DNA damage induced by combination treatment with irradiation and the compounds at a dose of 200nM was determined by induction of γH2AX+ foci. DNA damage is expressed as relative ratios of number of γH2AX+ cells at time points of 5 minutes and 2 hours after treatment divided by that of γH2AX+ cells without treatment by the compounds at each time point.
Induction of DNA DSB in ATL cells by NK314 or etoposide and comparison of DNA repair capacity in NK314- or etoposide-treated cells after irradiation. (A) After treatment with the indicated concentrations of NK-314 or etoposide, induction of γH2AX+ foci was determined by flow cytometry using an H2A.X Phosphorylation Assay Kit. The percentage of γH2AX+ foci was calculated by dividing by the number of cells without treatment by the compounds. (B) DNA damage induced by combination treatment with irradiation and the compounds at a dose of 200nM was determined by induction of γH2AX+ foci. DNA damage is expressed as relative ratios of number of γH2AX+ cells at time points of 5 minutes and 2 hours after treatment divided by that of γH2AX+ cells without treatment by the compounds at each time point.
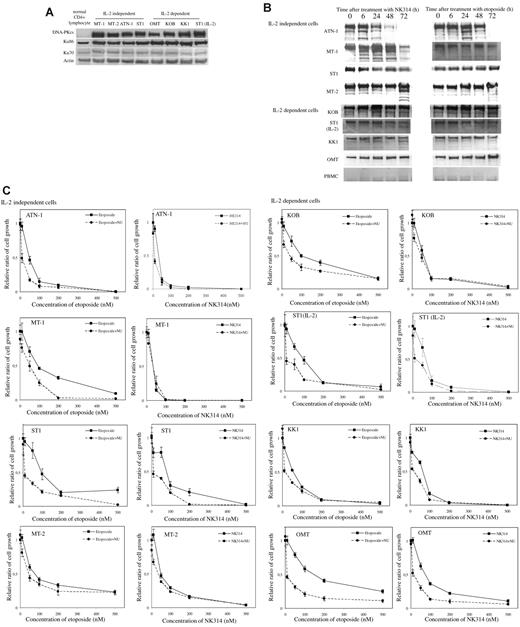
DNA-PKcs overexpressed in ATL cells, and NK314 induced protein degradation of DNA-PKcs
DNA-PK is a family of phosphoinositide 3-kinases and a major molecule involved in the repair of DNA DSBs after irradiation and genotoxic stimuli. We determined the amount of DNA-PK complex in ATL cell lines (Figure 4A). The DNA-PK complex consists of Ku86, Ku70, and DNA-PKcs. Expression of Ku proteins is ubiquitous, but DNA-PKcs levels were apparently high in all ATL cell lines compared with normal CD4+ T cells. NK314 induced degradation of DNA-PKcs in 5 of 8 cell lines but did not in OMT cells (Figure 4B). In the case of ST-1 (IL-2–independent) cells, protein fragmentation was not observed, but the amount of DNA-PKcs protein apparently decreased after treatment with NK314. After treatment with etoposide, the degradation of DNA-PKcs was not observed, except in ATN-1 cells; indeed, the amount increased in some cell lines (MT-1, IL-2–independent ST-1, and OMT cells). Ku86 and Ku70 were not altered by treatment with NK314 or etoposide in any ATL cell lines or normal PBMCs (supplemental Data). To clarify the contribution of DNA-PK to the effects of NK314, such as DNA damage response and cell growth inhibition, ATL cell lines were treated with the DNA-PK inhibitor NU7026 in combination with NK314 or etoposide. A total of 10μM of NU7026 alone did not inhibit growth of ATL cells (data not shown). Figure 4C showed combination effects of NU7026 with NK314 or etoposide on cell growth inhibition of ATL cell lines. In combination with etoposide, NU7026 enhanced cell growth inhibition compared with etoposide alone in all 8 cell lines, especially at lower doses. However, in combination with NK314, NU7026 showed enhancement of cell growth inhibition effects on some cell lines, but the effects were marginal (Figure 4C).
Degradation of DNA-PKcs induced by NK314 in ATL cells. (A) Expression of DNA-PK complex in various ATL cell lines and normal CD4+ lymphocytes was detected using Western blot analysis. The results with normal CD4+ cells were obtained from a different gel in the same experiments with the others. Vertical line(s) have been inserted to indicate a repositioned gel lane. (B) Degradation of DNA-PKcs was determined after treatment with NK314 or etoposide at each time point using Western blot analysis. (C) Contribution of DNA-PK to the inhibition of cell growth by NK314 in ATL cell lines. Cells were treated with etoposide or NK314 with or without NU7026 (NU), a DNA-PK inhibitor. Number of cells was determined with Cell Counting Kit-8. Relative ratio of cell growth was calculated by dividing by the number of untreated cells and expressed as mean ± SD of triplicate analyses.
Degradation of DNA-PKcs induced by NK314 in ATL cells. (A) Expression of DNA-PK complex in various ATL cell lines and normal CD4+ lymphocytes was detected using Western blot analysis. The results with normal CD4+ cells were obtained from a different gel in the same experiments with the others. Vertical line(s) have been inserted to indicate a repositioned gel lane. (B) Degradation of DNA-PKcs was determined after treatment with NK314 or etoposide at each time point using Western blot analysis. (C) Contribution of DNA-PK to the inhibition of cell growth by NK314 in ATL cell lines. Cells were treated with etoposide or NK314 with or without NU7026 (NU), a DNA-PK inhibitor. Number of cells was determined with Cell Counting Kit-8. Relative ratio of cell growth was calculated by dividing by the number of untreated cells and expressed as mean ± SD of triplicate analyses.
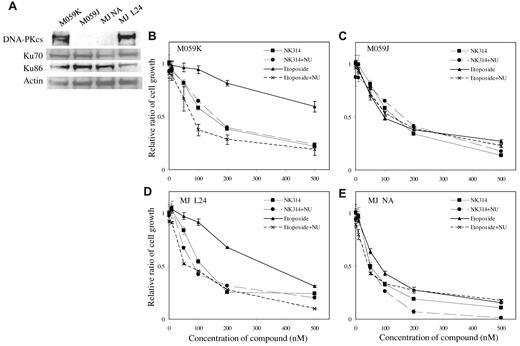
Next, we compared the effects of NK314 in a DNA-PKcs–deficient cell line and a parental cell line. The DNA-PK mutant glioblastoma cell line, M059J, did not express any DNA-PKcs (Figure 5A). Parental M059K cells expressed all subunits of DNA-PK complex and were used as a control. Both NK314 and etoposide inhibited cell growth of M059J with almost the same potency, but the effect of etoposide was remarkably weaker on M059K cells, having intact DNA-PKcs than that of NK314 (Figure 5B-C). These results suggest that DNA-PK is involved in sensitivity to cell toxicity induced by these Top2 inhibitors. Together with the results of induction of γH2AX+ cells, it suggests that NK314 possesses both Top2 inhibition (induction of DNA damage) and inhibition of DNA-PK activity (inhibition of DNA repair), resulting in potent cytotoxic effects on ATL cells. To examine this hypothesis, we studied the effect of NU7026 on M059J and M059K cells. Again, NU7026 alone showed no apparent cytotoxic effect on either M059J or M059K (data not shown). In combination with NU7026 and etoposide, NU7026 enhanced the inhibitory activity of etoposide against M059K cells (DNA-PKcs intact), but it did not against M059J cells (DNA-PK deficient) (Figure 5B-C). The additional effect of NU7026 in combination with NK314 was not prominent in either M059K or M059J. To confirm the contribution of DNA-PK to cell growth inhibition of M059 cells by NK314, we introduced DNA-PKcs cDNA into M059J cells (DNA-PKcs–deficient cell line) and established cell lines as reported previously.39 Among them, we used MJ L24 (DNA-PKcs cDNA introduced) and MJ NA (mock vector introduced) cells in this experiment. Expression of DNA-PKcs and Ku proteins in MJ L24 cells was confirmed by Western blotting (Figure 5A). The growth inhibitory activity of NK314 or etoposide on DNA-PK–deficient MJ NA cells was almost equal to parental M059J cells (Figure 5E). However, cell toxicity of etoposide in MJ L24 cells became weaker, and the effect was comparable with that of DNA-PKcs–intact M059K cells (Figure 5D). Furthermore, NU7026 enhanced cell toxicity in combination with etoposide in MJ L24 cells as shown on M059K cells (Figure 5D). These data clearly show that DNA-PK inhibition contributed to cell growth inhibition of M059 cells, and NK314 inhibits DNA-PK in addition to TopIIα.
Effect of NK314 on growth of M059 cell lines. (A) Expression of DNA-PK complex in M059K, M059J (a DNA-PKcs mutant), MJ NA (mock vector-transfected), and MJ L24 (DNA-PK cDNA-transfected) cells was detected by Western blot analysis. M059 cells were treated with etoposide or NK314 in combination with or without NU7026 (NU). Effects of NK314 or etoposide on cell growth of M059K (B), M059J (C), MJ L24 (D), or MJ NA (E) were determined with Cell Counting Kit-8. Relative ratio of cell growth was calculated by dividing by the number of untreated cells and expressed as mean ± SD of triplicate analyses.
Effect of NK314 on growth of M059 cell lines. (A) Expression of DNA-PK complex in M059K, M059J (a DNA-PKcs mutant), MJ NA (mock vector-transfected), and MJ L24 (DNA-PK cDNA-transfected) cells was detected by Western blot analysis. M059 cells were treated with etoposide or NK314 in combination with or without NU7026 (NU). Effects of NK314 or etoposide on cell growth of M059K (B), M059J (C), MJ L24 (D), or MJ NA (E) were determined with Cell Counting Kit-8. Relative ratio of cell growth was calculated by dividing by the number of untreated cells and expressed as mean ± SD of triplicate analyses.
NK314 inhibited the tumor growth of MT-1 cells transplanted into SCID mice
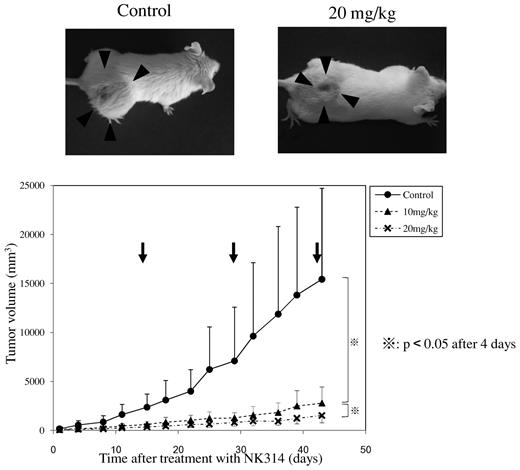
To examine the antitumor effects of NK314 against ATL cells in vivo, we established a mouse model carrying a xenograft tumor of ATL cells. Among several cell lines examined, MT-1 cells were stably transplantable to the subcutaneous tissue of SCID mice, so we used this model to evaluate antitumor activity of NK314 against ATL. After 4 Gy irradiation, MT-1 cells were inoculated into subcutaneous tissue of the mouse's back; and 14 days after inoculation, a visible tumor appeared. After that, NK314 was injected intraperitoneally once every 14 days. Figure 6 shows tumor growth curves for the MT-1 cells inoculated in the backs of the mice. We applied several experimental protocols using various NK314 doses and treatment schedules. Because treatment with more than 50 mg/kg of NK314 biweekly showed toxic effects, we used 2 doses (10 and 20 mg/kg) of NK314 biweekly. At a dose of 10 mg/kg, NK314 effectively inhibited tumor growth of MT-1 cells; and apparent side effects, such as body weight loss, myelosuppression, liver dysfunction, or renal insufficiency, were not observed (supplemental Data). We also studied the toxic effects of NK314 using colony-formation assay with normal human bone marrow cells. NK314 dose-dependently inhibited the colony formation of normal human bone marrow cells, but the inhibitory activity was comparable with, or slightly weaker than, that of etoposide (supplemental Data). These results suggest that biweekly treatment with NK314 at a dose of 10 mg/kg is efficient to inhibit growth of established ATL-cell tumors.
Effect of NK314 on tumor growth of MT-1 cells transplanted into SCID mice. MT-1 cells were injected into the dorsal flank of SCID mice after 4 Gy irradiation. NK-314 at a dose of 10 mg/kg (▴) or 20 mg/kg (×), or phosphate-buffered saline (●), was injected intraperitoneally at days 14, 28, and 42 after inoculation (arrows). Tumor volume was calculated as (short axis)2 × (long axis)/2.
Effect of NK314 on tumor growth of MT-1 cells transplanted into SCID mice. MT-1 cells were injected into the dorsal flank of SCID mice after 4 Gy irradiation. NK-314 at a dose of 10 mg/kg (▴) or 20 mg/kg (×), or phosphate-buffered saline (●), was injected intraperitoneally at days 14, 28, and 42 after inoculation (arrows). Tumor volume was calculated as (short axis)2 × (long axis)/2.
Discussion
Molecular targeting therapy is a sophisticated treatment strategy for some cancer types, including chronic myeloid leukemia, multiple myeloma, and lung cancer. In the case of ATL, several molecular targets associated with cellular transformation and disease progression have been identified that could be excellent targets for therapy. However, ATL cells display a variety of heterogeneous genetic alterations. It has been suggested that this complexity of genetic changes in ATL cells allows them to easily adapt to a single agent. Even though various combination chemotherapies using multiple anticancer drugs have been applied to the treatment of ATL, they have failed in that the median survival time is limited to only 13 months with the VCAP-AMP-VECP protocol.4 Therefore, we focused on investigating sensitization toward anticancer drugs or irradiation.
NK314, a new Top2α specific inhibitor, showed potent antitumor activity for ATL with in vitro and in vivo models. In addition to inhibition of Top2α, we found that NK314 inhibited DNA DSB repair mediated by inducing degradation of DNA-PKcs. DNA-PK is a member of the phosphoinositide 3-kinase family and a critical kinase for repair of DNA DSBs initiated with various genotoxic stresses, such as ionizing radiation, oxyradicals, and anticancer agents.27-29 Various agents for inhibiting DNA-PK have been identified and clinical trials have been conducted using both subclinical and clinical approaches.42-47 As for ATL cells, it was reported that transcription factor Tax binds to DNA-PKcs and activates DNA-PK. However, the Tax-DNA-PK complex was incapable of repairing new DNA DSBs induced by genotoxic stimuli in ATL cells.48,49 Considering these results, impairment of DNA-PK mediated DNA DSB repair could be associated with genetic instability and cell transformation in the Tax-dependent early stage of ATL, whereas overexpression of DNA-PK or elevated DNA-PK activity could be associated with multidrug-resistant phenotypes in overt ATL, as reported in other types of cancers.42-47 In our experiments, it was difficult to analyze the association of Tax expression and effect of NK314 or DNA-PK inhibitor on cell growth inhibition in ATL cells because only one cell line (MT-2) expressed a detectable amount of Tax protein (data not shown). Genetic instability is usually correlated with cell proliferation capacity, and many cancer cell types have a tendency to be genetically unstable. Therefore, impairment of DNA repair capacity in rapidly proliferating cancer cells may induce accumulation of lethal genetic changes, resulting in apoptosis. Indeed, various inhibitors of the DNA repair system can sensitize toward chemotherapy and radiation therapy; their anticancer effects have been investigated in the laboratory and in the subclinical situation.42-47
In this paper, we presented evidence that ATL cells express high amounts of DNA-PKcs, and a specific inhibitor of DNA-PK enhanced cell toxicity induced by etoposide. The results prompted us to use DNA-PK inhibitors in combination with anticancer drugs for treatment of ATL. However, the combination therapy poses other problems to be solved, such as bioavailability and side effects of each compound, as well as their interplay. Because NK314 functions dually as a potent inhibitor of Top2α and DNA-PK, monitoring of the aforementioned drug-drug interaction is not necessary. Another problem concerning use of a DNA-PK inhibitor is the occurrence of a second, therapy-related malignancy, such as leukemia or myelodysplastic syndrome, especially in combination with Top2 inhibitors, alkylating agents, and irradiation.50,51 In the literature, Top2α is more abundantly expressed in cancer cells and regulates the cell cycle.41,52 Inhibition of Top2α is responsible for cytotoxic effects on cancer cells, whereas inhibition of Top2β might influence the development of a second malignancy. These results suggest that NK314 has an advantage as a new type of anticancer agent in ATL treatment. On the other hand, the DNA-PK inhibitor NU7026 enhanced cytotoxicity of etoposide on ATL cells, but its effects varied among ATL cell lines and the combination effects of NU7026 for etoposide were slightly weaker on IL-2–dependent ATL cell lines than those on IL-2–independent cell lines. Although we have not clarified the mechanisms yet, it is possible that the IL-2 receptor signal activates the phosphoinositide 3 AKT kinase pathway in regulatory T cells, possible counterparts of ATL cells,53 ; thus, treatment with IL-2 on ATL cell lines might act as an antagonistic against a DNA-PK inhibitor.
As for the toxicity of the compounds, we monitored body weight change, myelosuppression, and hepatorenal toxicity in the in vivo experiment involving xenograft tumors. As shown in the supplemental Data, biweekly administration of a dose of 20 mg/kg NK314 showed no apparent hepatorenal toxicity and produced only moderate reduction of leukocytes (not statistically significant). We also investigated the toxicity of NK314 on in vitro cultured human bone marrow cells. The 50% inhibitory concentration of NK314 for colony formation of freshly isolated human bone marrow cells was approximately 100nM, which is comparable with etoposide. Because NK314 has more potent activity for cell growth inhibition of ATL cells, it offers promise for clinical application in the treatment of patients with aggressive ATL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kunitaka Shimotono at Kyoto University for his generous gift of the MT-2 cell line and Takeda Chemical Industries for providing the IL-2.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology and the Smoking Research Foundation.
Authorship
Contribution: T.H., A.S., R.T. M.I., N.S.-A., and E.S. performed the research and analyzed the data; N.S.-A. edited the paper; R.T., M.I., A.K., and S.K. provided the samples and related data; K.O. provided agents and fruitful discussion; and E.S. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eisaburo Sueoka, Department of Transfusion Medicine, Saga University Hospital, Saga, 849-8501, Japan; e-mail: sueokae@cc.saga-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal