In this issue of Blood, Farha and colleagues have identified a myeloproliferative process that is intrinsic to human pulmonary arterial hypertension (PAH), but appears coupled to pathologic vascular remodeling in the lung.1
Pulmonary arterial hypertension is a disorder of sustained elevation of pulmonary arterial pressure associated with vasoconstriction, vascular remodeling, and the presence of neovascular formations called plexiform lesions, composed of endothelial and myofibroblast cells. Patients may present with heritable PAH with germline mutations in BMPR2 in approximately 70% of subjects or with idiopathic PAH (without apparent family history) in whom 10% to 40% display the BMPR2 mutations.2 However, the BMPR2 mutations are not sufficient alone to explain all cases of PAH and other genetic or environmental factors must also play a role.
Because of abnormal vascular cell proliferation in PAH, it has been speculated that PAH may be a disease of “neoplastic” angiogenesis.3 Indeed, endothelial cells isolated from the lungs of patients with PAH are hyperproliferative and apoptosis-resistant, and display a metabolic switch to predominantly glycolytic pathways.3 These endothelial cells also display altered intracellular hypoxia sensing with enhanced HIF-1α and STAT3 activation supporting their proangiogenic phenotype.3 A recent report of acquired chromosomal abnormalities in pulmonary endothelial cells in > 50% of PAH patients (also present in plexiform lesions) supports a hypothesis for the neoplasia-like accumulation of somatic mutations occurring in all forms of PAH that may underlie the aberrant vascular phenotype.4
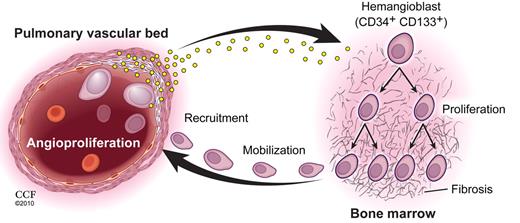
While some subsets of proangiogenic hematopoietic cells, including CD34+CD133+ cells, are vital promoters of angiogenesis in human health and disease,5 CD34+CD133+ progenitor cells have been reported to circulate in significantly greater numbers in patients with PAH and to correlate with severity of disease and to demonstrate greater proliferative potential.6 Farha and colleagues confirmed the higher circulating concentrations of CD34+CD133+ cells in PAH patients and also reported higher concentrations of these cells in patient bone marrowaspirates (see figure). In those marrow samples, CD34+CD133+ cells displayed higher clonogenic proliferation and increased myeloid colony forming activity. Bone marrow fibrosis was present to a greater degree in PAH subjects than in control subjects, and the combination of marrow fibrosis with elevated hematopoietic myeloid progenitor counts suggests a myeloproliferative process is existent in PAH patients.
Patients with PAH display increased circulating levels of HIF-inducible factors, some produced by pulmonary endothelial cells, that promote hematopoietic stem/progenitor cell proliferation in a bone marrow microenvironment predisposed to myeloproliferation and fibrosis. Local production of HIF-inducible factors by the pulmonary arterial endothelial cells recruit the mobilized hematopoietic subsets into the vessels leading to inappropriate vascular remodeling that eventually obliterates vascular perfusion through the pulmonary arterioles. Copyright 2010. Cleveland Clinic. All rights reserved. See the complete figure by Farha et al on page 3485.
Patients with PAH display increased circulating levels of HIF-inducible factors, some produced by pulmonary endothelial cells, that promote hematopoietic stem/progenitor cell proliferation in a bone marrow microenvironment predisposed to myeloproliferation and fibrosis. Local production of HIF-inducible factors by the pulmonary arterial endothelial cells recruit the mobilized hematopoietic subsets into the vessels leading to inappropriate vascular remodeling that eventually obliterates vascular perfusion through the pulmonary arterioles. Copyright 2010. Cleveland Clinic. All rights reserved. See the complete figure by Farha et al on page 3485.
Surprisingly, nearly 10-fold more CD34+CD133+ cells were isolated from the pulmonary artery samples from patients with PAH undergoing lung transplantation compared with donor lung samples (not used for transplantation). Erythropoietin (Epo), stem cell factor (SCFs), and hepatocyte growth factor (HGF) were present at elevated concentrations in the serum of PAH subjects compared with control subjects, though only EPO concentrations were related to any clinical parameters of disease severity. Endothelial cells harvested from the lungs of PAH patients undergoing transplantation secreted significantly higher concentrations of HGF and stromal-derived factor 1α (SDF-1α) than endothelial cells isolated from donor lungs (whether cultured in normoxia or hypoxia). Immunostaining of human lung tissue from patients with PAH provided evidence for enhanced HGF and SDF-1α expression in endothelial cells within plexiform lesions. The greater expression of these molecules in the pathologic lung tissue in association with the higher serum concentrations of HGF in PAH subjects and greater secretion of HGF and SDF-1α by the endothelial cells isolated from PAH patient lungs, implicates lung endothelial cells in PAH patients as mediators of molecules that may have recruited the elevated concentrations of CD34+CD133+ progenitor cells into the vessels of affected patients.
To determine whether the bone marrow myeloproliferative disorder may be intrinsic to PAH patients, Farha and colleagues recruited nonaffected family members of patients with familial PAH and confirmed normal echocardiographic findings without elevated pulmonary pressures in the family members. All 9 family members displayed elevated circulating concentrations of CD34+CD133+ progenitor cells (at levels similar to their affected relatives with PAH) and a significant increase in marrow fibrosis compared with healthy unrelated control subjects. All the peripheral blood indices were normal for the nonaffected family members. These results led the authors to conclude that a subclinical myeloproliferative process is intrinsic to at least the familial form of PAH.
Patients with myeloproliferative diseases often develop PAH, but this secondary form of PAH has been reported to resolve with treatment of the underlying myeloproliferative process.7 Essentially all forms of myeloproliferative disorders result from acquired somatic mutations in a hematopoietic stem/progenitor cell that results in clonal production of red blood cells, platelets, and granulocytes. Most patients with myeloproliferative disease display mutations in the tyrosine kinase JAK2 that leads to constitutive activation of downstream signaling pathways and dysregulated hematopoiesis.7 Farha and colleagues did not observe any subjects with PAH that displayed mutations in JAK2 or significantly altered signal transduction and activator of transcription 3 and 5 (STAT3; STAT5) activation in the patients' CD34+CD133+ derived colonies. However, prior studies have detected intrinsically high levels of STAT3 activation in pulmonary artery endothelial cells of PAH patient lung samples.
Increasing evidence supports an interdependence of bone marrow-derived hematopoietic cells and vascular endothelium in vascular development, homeostasis, repair, and dysregulation.5 The data presented by Farha and colleagues highlights a potential interdependence of dysregulated hematopoiesis and abnormal pulmonary vascular endothelial behavior that may play a key role in the development of PAH. At present some additional work must be done to fully understand the mechanisms leading to the disturbed hematopoietic and endothelial processes. Are there common somatic mutations that affect both the bone marrow hematopoietic elements and the pulmonary vascular endothelium or do mutations occur in one population and the consequences of those genetic events lead to altered cell behaviors that induce abnormal responses in the other lineage and in concert cause PAH? Fully elucidating the underlying molecular mechanisms that account for the abnormal cell behaviors described by Farha and colleagues may provide a new paradigm for PAH treatments. At present, currently available vasodilator therapies for PAH, although helpful in improving exercise tolerance and quality of life,8 are only moderately effective in improving survival.8 These therapies are targeted to ameliorate the physiologic consequences of the remodeled pulmonary arterial vasculature and probably do not directly alter the underlying defects in the pulmonary vascular endothelium or the putative myeloproliferative disorder. New therapies targeted at treating the proposed myeloproliferative disorder described by Farha and colleagues may disrupt and diminish ongoing angiogenic overstimulation in the pulmonary endothelial cells that lessen or even prevent the emergence of plexiform lesions in the arterioles.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal