Abstract
Aging is associated with a decline in B-lymphopoiesis in the bone marrow and accumulation of long-lived B cells in the periphery. These changes decrease the body's ability to mount protective antibody responses. We show here that age-related changes in the B lineage are mediated by the accumulating long-lived B cells. Thus, depletion of B cells in old mice was followed by expansion of multipotent primitive progenitors and common lymphoid progenitors, a revival of B-lymphopoiesis in the bone marrow, and generation of a rejuvenated peripheral compartment that enhanced the animal's immune responsiveness to antigenic stimulation. Collectively, our results suggest that immunosenescence in the B-lineage is not irreversible and that depletion of the long-lived B cells in old mice rejuvenates the B-lineage and enhances immune competence.
Introduction
The aging of the immune system involves many physiologic changes that are collectively referred to as immune senescence. These changes affect both the innate and the adaptive immune system, often resulting in an immunodeficient state that is currently incompletely understood. The most important immunologic manifestations in aging include poor responsiveness to new or evolving pathogens and reduced efficacy of vaccination.1,2 Primary causes of this immune incompetence are the decline in production of naive lymphocytes in the bone marrow (BM) and thymus, and the expansion and increased survival of antigen-experienced memory lymphocytes. The outcome of these changes is a marked reduction in the diversity of the peripheral B-cell repertoire and in the capacity of the body to mount protective antibody responses.3-7 Most attempts to enhance vaccination efficacy in aging are based on improving the delivery of antigen but have yielded limited success.8 Thus, although generation of a memory compartment constitutes an essential process for the function of the immune system throughout life, the expansion of this compartment in aging limits the ability of the immune system to respond to new antigenic challenges.
The B lineage undergoes dramatic age-related alterations in its cellular composition. Old mice show a marked decrease in the frequency of precursor B cells and in B-cell production in the BM, whereas in the periphery there is a significant reduction in naive follicular B cells, reduction in B cell diversity, and accumulation of long-lived antigen-experienced B cells.7,9,10 In addition, intrinsic defects have been shown in the response of B cells from aged mice or humans, including: decreased class switch recombination and expression of activation induced cytidine deaminase and E47,11 reduced activation and expression of phosphotyrosine kinases and protein kinase C,12 decreased expansion of B cells in response to antigen,13 and reduced number and volume of germinal centers.14 These findings may explain why in aging the antibody response to new antigens is poor in quality and in quantity.
The mechanisms underlying the cellular alterations in aging are unclear. Most existing data support the idea that hematopoietic stem cells (HSCs) acquire intrinsic defects with aging that suppress B lymphopoiesis.15-19 Accordingly, the peripheral B-cell compartment adapts to these changes by accumulating long-lived cells, thus maintaining the peripheral B-cell numbers unchanged.20 Another hypothesis is that age-related alterations in the B lineage are mediated by the accumulation of long-lived peripheral B cells with age, which suppresses B lymphopoiesis in the BM. This hypothesis stems from earlier studies showing that B lymphopoiesis in the BM of young/adult mice is enhanced after ablating the peripheral B-cell compartment by sublethal irradiation.21-23 A major implication of this hypothesis is that the potential to reactivate B lymphopoiesis exists in old mice, and the recent identification of a subpopulation of HSCs in the BM of old mice that retains an equivalent potential to differentiate into lymphoid and myeloid lineages24 supports this. Hence, the present study attempts to test the second hypothesis and to determine whether aging in the B lineage is mediated by the accumulating long-lived B cells.
Methods
Ethics statement
All mouse studies described in the present study were approved by the committee for the supervision of animal experiments at the Technion.
Mice
Mice used were C57Bl6 mice that are normal, or carrying a targeted conditional Baff-r locus (BAFF-RFL),25 transgenic for Mx-cre,26 R26R-EYFP transgene for the EYFP-Cre reporter system,27 or Balb/c transgenic for human CD20 (hCD20 Tg).28 The mice were housed and bred at the animal facility of the Faculty of Medicine, Technion. For the experiments, we considered old mice to be 20 months of age or older and young mice to be 3 to 4 months old. In addition, old mice were prescreened by staining of blood samples to ensure they have an “old-like” B-cell phenotype before treatment. An “old-like” B-cell phenotype in C57Bl6 mice and the hCD20Tg mice was determined when the frequency of newly generated AA4.1-expressing B cells in peripheral blood was less than 5% of the B220+ cells (supplemental Figure 4, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In some experiments, 3-83Tg mice29 were used and “old-like” B-cell phenotype in these mice was determined by staining of peripheral blood for the loss of transgenic receptor expression in more than 50% of the B cells using anti–3-83 idiotype antibody, as described by Johnson et al.9
B-cell depletion
To deplete B cells in vivo, mice were injected intraperitoneally with the following mixture of monoclonal antibodies at 150 μg/mouse each: rat anti–mouse CD19 (clone 1D3), rat anti–mouse B220 (clone RA36B2), and mouse anti–mouse CD22 (clone CY34). After 48 hours, the mice were injected with a secondary antibody mouse anti–rat κ (clone TIB216) at 150 μg/mouse. To deplete B cells in the hCD20 Tg model, mice were injected intraperitoneally with 1 mg/mouse of mouse anti–human CD20 monoclonal antibodies (clone 2H7) as described.28 Injected mice were bled from the tail vain at different time intervals to determine depletion efficiency and the kinetics of B-cell return by flow cytometry (supplemental Figure 5). In some experiments, mice were killed to determine depletion efficiency by flow cytometry and by absolute numbers in different lymphoid organs.
Flow cytometry
Single-cell suspensions of from BM, spleen, lymph node, peritoneal cavity, and peripheral blood were stained for surface marker expression using fluorescein isothiocyanate-, phycoerythrin-, allophycocyanin-, Pacific blue–, Cy5-, and biotin-conjugated antibodies, followed with streptavidin peridinin chlorophyll protein. Data were collected on a FACSCalibur (BD Biosciences, Immunocytometry Systems) or on FACSLSRII (BD Biosciences) and analyzed using the CELLQuest or FlowJo Version 8 software (TreeStar). Analysis of B-cell populations and hematopoietic progenitors was conducted as described.16,30
Immunization and poly I:C administration
To activate the Mx-cre in vivo for ablation of loxP-flanked loci, mice received intraperitoneal injections of 3 doses of polyriboinosinic acid/polyribocytidylic acid (poly I:C) (400 μg/mouse each time; InvivoGene) on days 0, 3, and 6. Deletion efficiency was determined by flow cytometry using the EYFP-Cre reporter system as described.27 In some experiments, mice were injected intraperitoneally with nitrophenyl-chicken γ-globulin (NP-CGG) (50 μg/mouse) in alum adjuvant. Mice were bled from the tail vain, and anti-NP IgG1 antibodies in serum were quantified by standard enzyme-linked immunosorbent assay using nitrophenyl-conjugated bovine serum albumin–coated plates.
Statistical analysis
The statistical significance of differences between experimental groups was determined using unpaired Student t test, with differences considered significant at P < .05.
Results
Chronic B-cell deficiency prevents the age-related decline of B lineage precursors in the BM
To study whether long-lived B cells mediate age-related decline in B lymphopoiesis, we first used a mouse model enabling us to ablate B cells and establish a chronic deficiency of peripheral B cells. For these experiments, we generated mice that are homozygous for a targeted loxP-flanked Baff-r allele (BAFF-RFL/FL)25 and transgenic for cre-recombinase driven by the type I interferon responsive promoter (Mx-cre,26 ). BAFF-BAFF-R signaling is essential for the survival of mature B cells but dispensable for B lymphopoiesis in the BM.25 In these mice, the administration of the interferon inducer poly I:C results in the ablation of BAFF-R (deletion efficiency in the spleen 80%-90% determined by EYFP-Cre-reporter system27 ; supplemental Figure 1C), the depletion of approximately 50% of the B cells as measured in the peripheral blood and spleen, and the appearance of many newly generated B220+/AA4.1+ B cells in peripheral blood and spleen (supplemental Figure 1A-B).
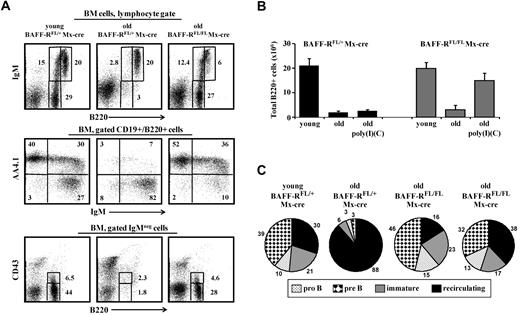
In old BAFF-RFL/FL/Mx-cre mice, the peripheral B-cell deficiency that was induced on the ablation of BAFF-R stimulated significant B lymphopoiesis in the BM that was similar to the B lymphopoiesis of young mice (Figure 1A). We found a high frequency of pro-B (B220+/CD43+/IgM−), pre-B (B220+/CD43−/IgM−), and immature B (B220+/IgM+) cells in the BM of old BAFF-RFL/FL/Mx-cre mice, but not of old control BAFF-RFL/+/Mx-cre mice, after treatment with poly I:C. Further quantification analysis of the B lineage subsets confirmed this finding. Thus, old BAFF-RFL/FL/Mx-cre mice treated with poly I:C for B-cell depletion had similar numbers of B220+ cells in the BM as those found in the BM of young mice (Figure 1B), with approximately 75% of them being newly generated and residing in the BM (∼ 15% pro-B, ∼ 38% pre-B, ∼ 20% immature) and only 25% recirculating (B220hi/IgM+/AA4.1−; Figure 1C right, 2 pie charts). In contrast, approximately 10-fold less B cells were found in the BM of the old, poly I:C-treated, control BAFF-RFL/+/Mx-cre mice (Figure 1B), and 88% of them were recirculating (Figure 1C), which is in agreement with previous studies that showed an age-related decline in the number of precursor B cells in the BM.10,20,31 A similar low number of B cells (3 × 106 ± 2 × 106) was detected in the BM of old, untreated BAFF-RFL/FL Mx-cre mice (Figure 1B), indicating the development of age-related changes in B lymphopoiesis in these mice. The poly I:C treatment had no effect on B lymphopoiesis in the young BAFF-RFL/FL/Mx-cre mice (not shown).
Ablation of BAFF-R induces chronic B-cell deficiency and prevents aging of B lymphopoiesis in the BM of old mice. Young (4 months) and old (20 months) C57Bl6 BAFF-RFL/FL/Mx-cre and young and old (age-matched) control C57Bl6 BAFF-RFL/+/Mx-cre mice received intraperitoneal injections of 3 doses of poly I:C (400 μg/mouse each time) to ablate the loxP-flanked BAFF-R allele. Before treatment, old mice were prescreened to have less than 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype. After 90 days, BM was analyzed for B lymphopoiesis. (A) BM cells were stained and analyzed by fluorescence-activated cell sorter (FACS) with a lymphocyte gate as defined by light scatter. The IgM versus AA4.1 plots were gated for CD19+/B220+ cells, and the B220 versus CD43 plots were gated on IgM− cells. (B) Absolute numbers of CD19+/B220+ cells in the BM of the indicated mice. The B-cell numbers were calculated based on the total number of nucleated cells purified from 2 femurs and 2 tibias (n = 3 mice in each group). (C) Quantification of B-cell subsets in the BM. The relative proportion of each B-cell subset was determined in the B220-gated population. Shown are representative results of young (n = 3) and old (n = 3) control BAFF-RFL/+/Mx-cre mice and 2 different Old BAFF-RFL/FL/Mx-cre mice (from n = 3).
Ablation of BAFF-R induces chronic B-cell deficiency and prevents aging of B lymphopoiesis in the BM of old mice. Young (4 months) and old (20 months) C57Bl6 BAFF-RFL/FL/Mx-cre and young and old (age-matched) control C57Bl6 BAFF-RFL/+/Mx-cre mice received intraperitoneal injections of 3 doses of poly I:C (400 μg/mouse each time) to ablate the loxP-flanked BAFF-R allele. Before treatment, old mice were prescreened to have less than 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype. After 90 days, BM was analyzed for B lymphopoiesis. (A) BM cells were stained and analyzed by fluorescence-activated cell sorter (FACS) with a lymphocyte gate as defined by light scatter. The IgM versus AA4.1 plots were gated for CD19+/B220+ cells, and the B220 versus CD43 plots were gated on IgM− cells. (B) Absolute numbers of CD19+/B220+ cells in the BM of the indicated mice. The B-cell numbers were calculated based on the total number of nucleated cells purified from 2 femurs and 2 tibias (n = 3 mice in each group). (C) Quantification of B-cell subsets in the BM. The relative proportion of each B-cell subset was determined in the B220-gated population. Shown are representative results of young (n = 3) and old (n = 3) control BAFF-RFL/+/Mx-cre mice and 2 different Old BAFF-RFL/FL/Mx-cre mice (from n = 3).
These results indicate that, when long-lived B cells do not accumulate, frequencies of precursor B cells in the BM do not significantly decline with age, thereby ensuring a continuous flow of B cells to the periphery. This may imply the existence of a feedback cross-talk mechanism between the B cells in the periphery and progenitors in the BM, which regulates B lymphopoiesis in aging.
Active B-cell depletion rejuvenates the B lineage in old WT mice
Because our results implicated the age-related decline in B lymphopoiesis with the accumulating population of long-lived B cells, we next tested whether active depletion of these cells could revive B lymphopoiesis in aged wild-type (WT) mice. In these experiments, we used a mixture of antibodies to deplete the B cells in vivo and followed the reconstitution of the peripheral B-cell compartment by analyzing peripheral blood samples over time. With this treatment, we demonstrate an 80%-90% depletion of the peripheral B cells, as analyzed in peripheral blood, spleen, peritoneal cavity, and lymph nodes (supplemental Figure 2B-C). A significant advantage of this depletion approach is the fact that most of the primary antibodies in this mixture (specific for CD19 and B220) are derived from rat and are rapidly cleared when injected into mice (within 5 days, supplemental Figure 2A). Moreover, this antibody depletion mixture does not substantially modify total numbers of developing B cells (pro-B, pre-B, and immature) and their relative frequency in the BM (supplemental Figures 3A and B, respectively). Hence, the rapid clearance of the antibody mixture and its effectiveness in selective depletion of peripheral B cells, but not progenitor B cells in the BM, indicate that the process of B lymphopoiesis in the injected mice is not repressed. This conclusion is also supported by the fact that young mice rapidly reconstitute the B cells in peripheral blood and spleen after depletion (supplemental Figure 2D-E).
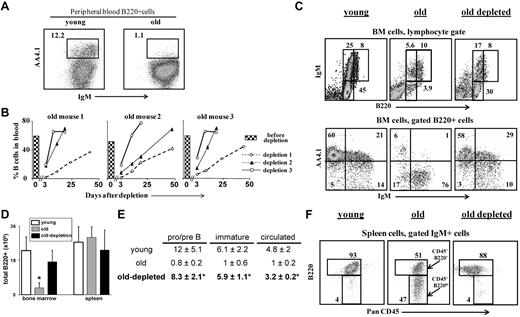
The results in Figure 2B show that reconstitution of B cells in the peripheral blood of old mice after B-cell depletion took more than 50 days. This slow reconstitution time reflected the poor B lymphopoiesis in the aged BM, as was also reported after lymphoablation by irradiation or treatment with cyclophosphamide,9,19 or in BM chimeras.15 To find whether the chronic B-cell deficiency had reactivated B lymphopoiesis, these old mice were subjected to second and third rounds of B-cell depletion (each round of depletion was introduced only after > 80% reconstitution of the B cells in peripheral blood). The results in Figure 2B reveal a profound decrease in the reconstitution time with each additional depletion circle. Thus, the reconstitution time of the B cells after the second round of depletion was decreased by more than 70%, and full reconstitution was established within 18 to 30 days. After the third round of depletion, the reconstitution time was decreased by 85%, achieving complete B-cell reconstitution within only 8 days (Figure 2B), which is similar to the time of B-cell return in young WT mice (supplemental Figure 2D).
B-cell depletion revives the B lineage in aged mice. Old B10D2 (20 months) WT mice (n = 5) were subjected to 3 rounds of B-cell depletion. In each round, mice were first injected intraperitoneally (day 0) with a mixture of monoclonal antibodies specific to B220 (150 μg/mouse), CD19 (150 μg/mouse), and CD22 (150 μg/mouse), followed by a second intraperitoneal injection of rat anti-mouse κ monoclonal antibodies (150μg/mouse) 48 hours later (more details on the effectiveness and the specificity of the depletion is detailed shown in supplemental Figure 2). Blood samples were collected at various times after depletion and analyzed for B-cell frequency (% B220+/CD19+ cells). The second and the third rounds were administered to each mouse only after more than 80% of the initial B-cell numbers had been restored. (A) Peripheral blood staining of representative young and old mice. Before treatment, old mice were prescreened to have less than 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype (see also supplemental Figure 4). (B) Kinetics of B-cell reconstitution after depletion. The time course of B-cell return after each depletion cycle is shown for 3 (of 5) individual mice. The histogram in each plot corresponds to the B-cell frequency in peripheral blood before the first depletion. (C) FACS analysis of the B lineage cells in the BM of mice subjected to 3 rounds of depletion. Mice were analyzed 10 to 14 days after restoring 100% of the B-cell counts in peripheral blood (100-120 days after first depletion was administered). BM cells were stained and analyzed by FACS with a lymphocyte gate as defined by light scatter. The IgM versus AA4.1 plot (bottom) was gated for B220+ cells. Representative plots of 5 mice are shown. (D-E) Absolute numbers of B220+ cells in BM and spleen (D), and of specific B-cell populations in the BM (E). Values are mean ± SD (× 106). The B-cell numbers were calculated based on the total number of nucleated cells purified from 2 femurs and 2 tibias (n = 5 mice in each group). *Significant difference (P < .05). (F) FACS analysis of the B lineage cells in the spleen of mice subjected to 3 rounds of depletion. Spleen cell analysis for B220 and PanCD45 was conducted on gated IgM+ cells. Gates pointed by arrows are for the PanCD45+/B220+ and the PanCD45+/B220lo populations. Representative plots of 5 mice are shown.
B-cell depletion revives the B lineage in aged mice. Old B10D2 (20 months) WT mice (n = 5) were subjected to 3 rounds of B-cell depletion. In each round, mice were first injected intraperitoneally (day 0) with a mixture of monoclonal antibodies specific to B220 (150 μg/mouse), CD19 (150 μg/mouse), and CD22 (150 μg/mouse), followed by a second intraperitoneal injection of rat anti-mouse κ monoclonal antibodies (150μg/mouse) 48 hours later (more details on the effectiveness and the specificity of the depletion is detailed shown in supplemental Figure 2). Blood samples were collected at various times after depletion and analyzed for B-cell frequency (% B220+/CD19+ cells). The second and the third rounds were administered to each mouse only after more than 80% of the initial B-cell numbers had been restored. (A) Peripheral blood staining of representative young and old mice. Before treatment, old mice were prescreened to have less than 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype (see also supplemental Figure 4). (B) Kinetics of B-cell reconstitution after depletion. The time course of B-cell return after each depletion cycle is shown for 3 (of 5) individual mice. The histogram in each plot corresponds to the B-cell frequency in peripheral blood before the first depletion. (C) FACS analysis of the B lineage cells in the BM of mice subjected to 3 rounds of depletion. Mice were analyzed 10 to 14 days after restoring 100% of the B-cell counts in peripheral blood (100-120 days after first depletion was administered). BM cells were stained and analyzed by FACS with a lymphocyte gate as defined by light scatter. The IgM versus AA4.1 plot (bottom) was gated for B220+ cells. Representative plots of 5 mice are shown. (D-E) Absolute numbers of B220+ cells in BM and spleen (D), and of specific B-cell populations in the BM (E). Values are mean ± SD (× 106). The B-cell numbers were calculated based on the total number of nucleated cells purified from 2 femurs and 2 tibias (n = 5 mice in each group). *Significant difference (P < .05). (F) FACS analysis of the B lineage cells in the spleen of mice subjected to 3 rounds of depletion. Spleen cell analysis for B220 and PanCD45 was conducted on gated IgM+ cells. Gates pointed by arrows are for the PanCD45+/B220+ and the PanCD45+/B220lo populations. Representative plots of 5 mice are shown.
The profound reduction in the time of B-cell return after multiple depletions suggested that the depletion of peripheral B cells and the consequential chronic B-cell deficiency had stimulated enhanced B lymphopoiesis in the BM of the old mice. Indeed, analysis of the BM of the treated mice revealed that B lymphopoiesis had been reactivated as revealed by the increase in absolute number of B220+ cells, relative to the number of B220+ cells in the BM of the old untreated mice (Figure 2D). Further analysis revealed a revival of B lymphopoiesis in the BM of old mice that had been subjected to multiple rounds of B-cell depletion, with profound increase in the frequencies and absolute numbers of pro-B/pre-B and immature B cells (Figure 2C,E). The increase in precursor frequencies was further confirmed by AA4.1 staining (Figure 2C bottom panel). These frequencies and absolute number of precursor B cells were similar to those in the BM of young mice (Figure 2C,E).
In the spleen, we found that the treated old mice completely restored the size of the B-cell population as revealed by the absolute B-cell numbers (Figure 2D). Moreover, Johnson et al have shown an accumulation of antigen-experienced long-lived B cells bearing the phenotype PanCD45+/B220lo in the spleen of old WT mice9 (Figure 2F middle panel, arrows indicating the antigen-experienced PanCD45+/B220lo population relative to the mature PanCD45+/B220+ population). Figure 2F shows that the PanCD45+/B220lo cells were eliminated in the old mice treated for B-cell depletion and replaced with a population of mature B cells that are PanCD45+/B220+. These observations indicate that the potential to produce a high output of B lymphocytes is retained in the aged BM and that the rate of B lymphopoiesis is dictated by long-lived peripheral B cells.
B-cell depletion stimulates expansion of early hematopoietic progenitor cell populations to reactivate B lymphopoiesis in the BM
To further understand at which stage of BM development aging was reversed by B-cell depletion, we analyzed different populations of hematopoietic progenitor cells in young, old, and old B-cell depleted mice. To do so, we used transgenic mice expressing human CD20 specifically in the B lineage (hCD20 Tg mice), starting from the late pre-B stage.28 A significant advantage of using these mice is that effective long-term chronic depletion of peripheral B cells is obtained by a single injection of mouse anti–human monoclonal antibodies specific to human CD20, without causing any depressing effect on B lymphopoiesis in the BM or resulting in major alterations in T-cell subsets in the periphery, as had been described.28,32 The use of these mice is also important to validate our results obtained in the BAFF-RFL/FL/Mx-cre and in WT mice treated with B cell–depleting antibody mixture, in a third experimental setting.
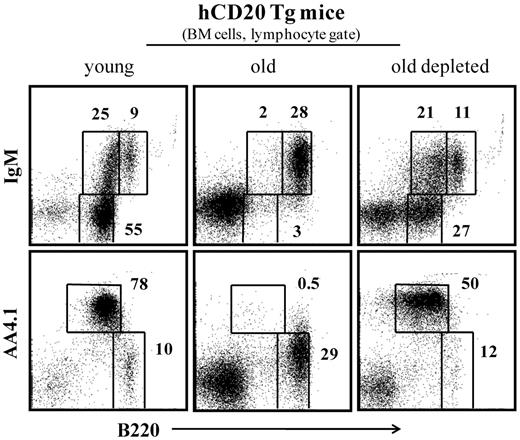
After B-cell depletion, complete B-cell reconstitution in hCD20 Tg mice lasted 60 to 70 days, as described.28,32 Figure 3 indicates that, as observed in the previous experimental settings shown in Figures 1 and 2, the long-term peripheral B-cell deficiency that was induced in old hCD20 Tg mice stimulated significant B lymphopoiesis in the BM. Thus, although most of the B220+ cells in the BM (lymphocyte gate) of old hCD20 Tg mice are circulated (B220hi/IgM+/AA4.1−), in old hCD20 Tg mice treated for B-cell depletion, there is a profound increase of up to 10-fold in frequencies of precursor B cells (pro-B/pre-B B220+/IgM−/AA4.1+ and immature B220+/IgM+/AA4.1+).
B-cell depletion in old hCD20 Tg mice reactivates B lymphopoiesis in the BM. Old (20 months) hCD20 Tg Balb/c mice (prescreened to have < 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype) were injected intraperitoneally with 1 mg/mouse of mouse antihuman CD20 monoclonal antibodies (clone 2H7). B-cell depletion was confirmed by staining of peripheral blood 5 days later. Mice were followed for 70 days until > 90% reconstitution of peripheral blood B cells was achieved. Mice were then analyzed by flow cytometry for the B lineage in the BM and compared with old (age-matched) and young (4 months old) hCD20 Balb/c controls). The BM cells were analyzed with a lymphocyte gate as defined by light scatter. Plots shown are representative of 4 mice in each group.
B-cell depletion in old hCD20 Tg mice reactivates B lymphopoiesis in the BM. Old (20 months) hCD20 Tg Balb/c mice (prescreened to have < 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype) were injected intraperitoneally with 1 mg/mouse of mouse antihuman CD20 monoclonal antibodies (clone 2H7). B-cell depletion was confirmed by staining of peripheral blood 5 days later. Mice were followed for 70 days until > 90% reconstitution of peripheral blood B cells was achieved. Mice were then analyzed by flow cytometry for the B lineage in the BM and compared with old (age-matched) and young (4 months old) hCD20 Balb/c controls). The BM cells were analyzed with a lymphocyte gate as defined by light scatter. Plots shown are representative of 4 mice in each group.
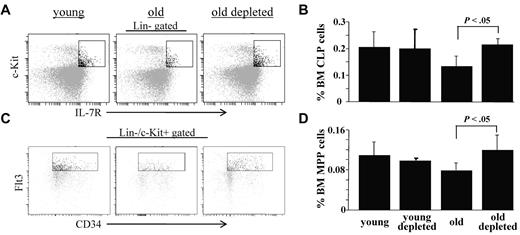
The frequencies of common lymphoid progenitors (CLPs, Lin−/c-Kit+/IL-7R+) and multipotent primitive progenitors (MPPs, Lin−/c-Kit+/CD34+/Flt3+) in the BM of these mice were also studied by flow cytometry. In agreement with earlier studies,10,16,33 we found that both of these populations are decreased in BM of old mice. In contrast, we found a significant increase in the frequencies of CLPs (Figure 4A-B) and MPPs (Figure 4B-C) in BM of the B-cell depleted old mice, to levels that are similar to those found in young mice. Thus, we conclude that peripheral B-cell depletion and the consequential induction of chronic B-cell deficiency stimulate expansion of early BM progenitor cell populations to reactivate B lymphopoiesis in aged mice.
B-cell depletion expands populations of BM hematopoietic progenitor cells in old mice. Old (20 months) hCD20 Tg Balb/c mice (prescreened to have < 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype) were treated for B-cell depletion by intraperitoneal injection of mouse anti–human CD20 monoclonal antibodies (clone 2H7). BM cells were analyzed by flow cytometry 70 days later for the frequencies of Lin−/c-Kit+/IL7R+ cells (CLPs) and Lin−/c-Kit+/CD34+/Flt3+ cells (MPPs) and compared with old (age-matched) and young (4 months old) hCD20 Balb/c controls. (A) Representative FACS profiles of the indicated mice showing CLPs. (B) BM frequencies of CLPs from the young, old, and old-depleted mice (n = 4 in each group). (C) Representative FACS profiles of the indicated mice showing MPPs. (D) BM frequencies of MPPs from the young, old, and old-depleted mice (n = 4 in each group). Statistically different differences are specified.
B-cell depletion expands populations of BM hematopoietic progenitor cells in old mice. Old (20 months) hCD20 Tg Balb/c mice (prescreened to have < 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype) were treated for B-cell depletion by intraperitoneal injection of mouse anti–human CD20 monoclonal antibodies (clone 2H7). BM cells were analyzed by flow cytometry 70 days later for the frequencies of Lin−/c-Kit+/IL7R+ cells (CLPs) and Lin−/c-Kit+/CD34+/Flt3+ cells (MPPs) and compared with old (age-matched) and young (4 months old) hCD20 Balb/c controls. (A) Representative FACS profiles of the indicated mice showing CLPs. (B) BM frequencies of CLPs from the young, old, and old-depleted mice (n = 4 in each group). (C) Representative FACS profiles of the indicated mice showing MPPs. (D) BM frequencies of MPPs from the young, old, and old-depleted mice (n = 4 in each group). Statistically different differences are specified.
B-cell depletion rejuvenates the peripheral B-cell repertoire in aged mice
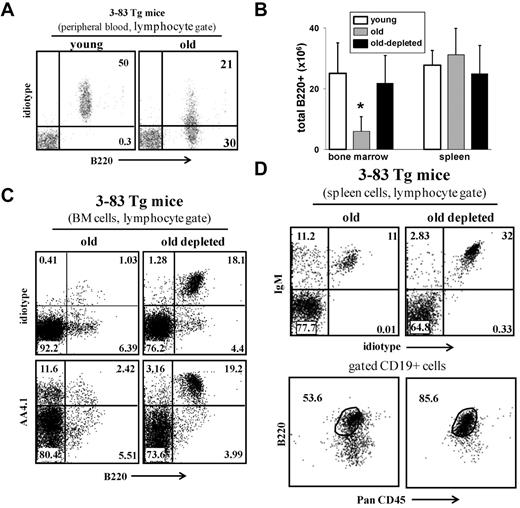
We next tested whether B-cell depletion rejuvenates the peripheral repertoire. Johnson et al used an immunoglobulin transgenic (Ig-Tg) mouse model (3-83Tg mice29 ) to measure age-associated changes in the B-cell repertoire.9 In young 3-83Tg mice, approximately 90% of the splenic B cells express the transgenic receptor.29 This peripheral repertoire changes with aging because of selection of long-lived cells bearing nontransgenic receptors, and in old 3-83Tg mice, it is dominated by B cells that express endogenous Ig genes9 (Figure 5A). Similar to the old WT mice, we found that B-cell depletion revived B lymphopoiesis in the old 3-83Tg mice, as revealed by the significant increase in absolute number of B cells in the BM (Figure 5B) and by the more than 15-fold increase in the frequency of newly generated B220+/AA4.1+ cells that express the transgenic receptor (stained with specific anti-idiotype antibody, ID+) in the BM (Figure 5C). In the spleen of the treated mice, we found that most of the reconstituting B cells (> 90%) express the transgenic receptor and that the long-lived PanCD45+/B220lo compartment had been replaced with a population of mature B cells that are PanCD45+/B220+ (Figure 5D). We conclude that elimination of the long-lived antigen-experienced B cells in old 3-83Tg mouse reactivates B lymphopoiesis in the BM to reconstruct a new Ig-Tg peripheral repertoire that is not different from that of a young 3-83Tg mouse.29
B-cell depletion in old 3-83Tg mice revives B lymphopoiesis in the BM and rejuvenates the peripheral repertoire. Old B10D2 3-83Tg mice (20 months, confirmed to have an old-like B-cell phenotype by blood-sample staining) were treated for B-cell depletion. This was achieved by an initial intraperitoneal injection with a mixture of monoclonal antibodies specific to B220 (150 μg/mouse), CD19 (150 μg/mouse), and CD22 (150 μg/mouse), followed by a second intraperitoneal injection of rat anti–mouse κ monoclonal antibodies (150 μg/mouse) 48 hours later. Mice were bled 3 days after last injection to ensure more than 90% B-cell depletion in peripheral blood. After 65 days, the BM and spleen were analyzed for the B lineage by flow cytometry. (A) Peripheral blood staining of representative young and old 3-83Tg mice. Old mice were prescreened to have less than 50% B cells in peripheral blood expressing the transgenic receptor. (B) Absolute numbers of B220+ cells were calculated based on the total number of nucleated cells purified from the BM (2 femurs and 2 tibias) or spleen (n = 4 mice in each group). *Significant difference (P < .05). (C) BM cells were analyzed by flow cytometry with a lymphocyte gate as defined by light scatter. Expression of the transgenic receptor was detected using an anti–3-83 clonotype specific monoclonal antibody 54.1. (D) Spleen cells from the indicated mice were analyzed by flow cytometry. Analysis for B220 and PanCD45 was conducted on gated CD19+ cells. The plots shown are representative of 4 mice in each group.
B-cell depletion in old 3-83Tg mice revives B lymphopoiesis in the BM and rejuvenates the peripheral repertoire. Old B10D2 3-83Tg mice (20 months, confirmed to have an old-like B-cell phenotype by blood-sample staining) were treated for B-cell depletion. This was achieved by an initial intraperitoneal injection with a mixture of monoclonal antibodies specific to B220 (150 μg/mouse), CD19 (150 μg/mouse), and CD22 (150 μg/mouse), followed by a second intraperitoneal injection of rat anti–mouse κ monoclonal antibodies (150 μg/mouse) 48 hours later. Mice were bled 3 days after last injection to ensure more than 90% B-cell depletion in peripheral blood. After 65 days, the BM and spleen were analyzed for the B lineage by flow cytometry. (A) Peripheral blood staining of representative young and old 3-83Tg mice. Old mice were prescreened to have less than 50% B cells in peripheral blood expressing the transgenic receptor. (B) Absolute numbers of B220+ cells were calculated based on the total number of nucleated cells purified from the BM (2 femurs and 2 tibias) or spleen (n = 4 mice in each group). *Significant difference (P < .05). (C) BM cells were analyzed by flow cytometry with a lymphocyte gate as defined by light scatter. Expression of the transgenic receptor was detected using an anti–3-83 clonotype specific monoclonal antibody 54.1. (D) Spleen cells from the indicated mice were analyzed by flow cytometry. Analysis for B220 and PanCD45 was conducted on gated CD19+ cells. The plots shown are representative of 4 mice in each group.
Rejuvenated B lineage in old mice confers an enhanced immune responsiveness
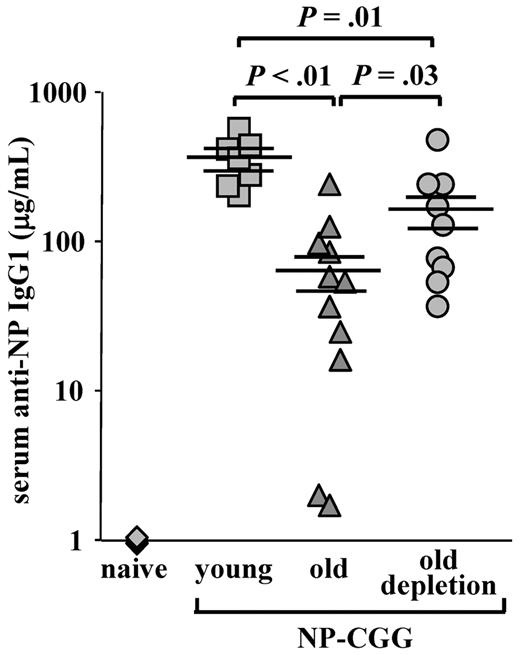
One of the most important changes in aging is the failure to mount protective antibody responses to vaccination and to infectious agents because of a reduction in the diversity of the peripheral repertoire.3,5,7 Because the peripheral compartment of the aged mice was replaced and rejuvenated after B-cell depletion, we next tested whether this new compartment conferred an increased competence to mount an antibody response against a new antigenic challenge. As shown in Figure 6, old WT mice produced a very poor anti-NP IgG1 response compared with young mice (66.5 ± 19.8 relative to 347.3 ± 47.7, P < .01). In contrast, a significant 3- to 4-fold increase in anti-NP IgG1 antibody titers was found in old WT mice that were subjected to B-cell depletion (161.3 ± 44.8 in old depleted relative to 66.5 ± 19.8 in the old, P = .03). These levels, however, were still lower than those of the young mice (P = .01). Hence, the reconstituted B-cell compartment in old mice restored in part the capacity to mount an antibody response to a new antigenic challenge.
Old mice with a rejuvenated peripheral repertoire mount an increased anti-NP IgG1 response. Old C57Bl6 WT mice (22 months, prescreened to have < 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype) were subjected to one round of B-cell depletion and were immunized intraperitoneally with NP-CGG 70 days later. Before immunization, these mice were bled to confirm reconstitution of >80% of B220+ in peripheral blood. Old, age-matched, and young C57Bl6 mice (4 months) that were untreated for B-cell depletion were used as controls and immunized with NP-CGG at the same time. The amount of anti–NP-specific IgG1 antibodies in the serum was determined by enzyme-linked immunosorbent assay 7 days later, using an IgG1 standard curve for reference. The plot shows titers of anti-NP IgG1 antibodies of individual mice (old depleted, n = 9; old untreated, n = 11; young untreated, n = 7; and naive, n = 4) collected from 4 different experiments and run simultaneously, and the mean and SEs of each group. Student t test was conducted to examine statistical significance between antibody titers of different groups.
Old mice with a rejuvenated peripheral repertoire mount an increased anti-NP IgG1 response. Old C57Bl6 WT mice (22 months, prescreened to have < 5% AA4.1+ B cells in peripheral blood as an indication for an “old-like” B-cell phenotype) were subjected to one round of B-cell depletion and were immunized intraperitoneally with NP-CGG 70 days later. Before immunization, these mice were bled to confirm reconstitution of >80% of B220+ in peripheral blood. Old, age-matched, and young C57Bl6 mice (4 months) that were untreated for B-cell depletion were used as controls and immunized with NP-CGG at the same time. The amount of anti–NP-specific IgG1 antibodies in the serum was determined by enzyme-linked immunosorbent assay 7 days later, using an IgG1 standard curve for reference. The plot shows titers of anti-NP IgG1 antibodies of individual mice (old depleted, n = 9; old untreated, n = 11; young untreated, n = 7; and naive, n = 4) collected from 4 different experiments and run simultaneously, and the mean and SEs of each group. Student t test was conducted to examine statistical significance between antibody titers of different groups.
Discussion
The present study shows that B lymphopoiesis can be reactivated in aging, thus indicating that aging in the B lineage is not an irreversible process. Our data support the idea that in aged mice there is a feedback mechanism between the peripheral B-cell compartment and the progenitor compartment in the BM in controlling B-cell output and its persistence. Earlier studies to demonstrate such a feedback mechanism in the B lineage used young (2- to 3-month-old) mice and came to controversial conclusions. Thus, Osmond et al,21 Park and Osmond,23 and Cancro et al22 demonstrated homeostatic regulation, whereas Agenes et al34 suggested that B-cell production in the BM is autonomously regulated. The results and conclusions of the present study do not support or contradict any of these previous works because our study was conducted in old mice, where dramatic alterations in the peripheral B-cell compartment develop physiologically, including accumulation of long-lived memory cells and a significant reduction in naive follicular B cells.7,9,10 Hence, this feedback mechanism may develop with age and may not be relevant to young mice used in the earlier studies.21-23,34
We show here that, when long-lived B cells do not accumulate, B lymphopoiesis does not decrease significantly with age. Moreover, on elimination of peripheral B cells in aged mice, B lymphopoiesis in the BM is reactivated, indicating that the long-lived peripheral B cells mediate the age-related alterations in B lymphopoiesis. This conclusion is validated here in 3 different experimental settings: BAFF-RFL/FL/Mx-cre, WT mice treated with B cell–depleting antibody mixture, and hCD20 Tg mice. The results we show here very well fit with the concept of hematopoiesis in which early cell lineages adapt their output to demand.35,36 There are several examples of homeostatic regulations in the hematopoietic system that do not substantially change with aging, including the effect of granulocyte colony-stimulating factor on proliferation and mobilization of stem cells,37 red blood cell production during physiologic and pathologic changes,36 and the ability of BM precursors to generate well-functioning CD4 T cells in mice.38 Thus, in the 3 experimental settings used here, the chronic demand or the acutely induced demand for B cells in the periphery prevented or reversed age-related alterations in the B lineage and imposed continuous B lymphopoiesis in the BM of old mice.
We show here that chronic B-cell deficiency, imposed by depletion of the peripheral B cells, reverses age-related alterations in hematopoietic progenitor content in aged BM. Earlier studies have shown that frequencies and differentiation of MPPs and CLPs are decreased in old mice,10,16,33,39 providing an explanation to the reduced production of B and T lymphocytes in aging. On B-cell depletion, however, we found expansion of these progenitor cell populations and reactivation of B lymphopoiesis. Hence, our results indicate plasticity in the size of CLP and MPP populations, which is determined by the peripheral demand for B cells in aging. Other studies, however, have shown that age-related alterations in the hematopoietic progenitor content in the BM and in the B lineage reflect acquisition of intrinsic defects15,16,18 and DNA damage in HSCs,17 which is a progressive and irreversible process.15,19 We propose that acquisition of these defects in HSCs may be regulated by the peripheral demands for B cells. For example, the decreased demand for B-cell production in aged mice resulting from the accumulation of long-lived cells may lead to selection of mutated slow cycling HSCs, whereas chronic demand for B cells enforces continuous B-cell production, and this may select for rapid cycling, unmutated HSCs.
However, the finding that early progenitor populations (MPPs and CLPs) can reexpand and that B lymphopoiesis can be reactivated in aging indicates that, even if HSCs do accumulate defects, these defects do not abolish the potential to produce B lineage cells in the old BM. This may suggest that low frequency of unmutated HSCs exists in the old BM and is selected to expand and differentiate into B lineage cells on B-cell depletion. Although the restoration of B lymphopoiesis in the aged mice may not occur rapidly, a long-term deficiency may be necessary to measurably accomplish it. This possibility is supported by recent studies proposing that age-related alterations in the B lineage are associated with changes in the clonal composition of the HSCs, rather than with changes in individual stem cells40 or dictated by clonal expansion of the functionally distinct HSC population.24 In agreement with this, we suggest that long-term deficiency may be necessary for expansion of unmutated, lymphoid-biased HSCs to measurably detect expansion of MPP and CLP populations and reactivation of B lymphopoiesis in old mice in vivo, as we show here.
The results described here are in apparent contradiction with earlier studies showing that BM or purified HSCs from old mice failed to reconstitute the peripheral B-cell compartment on short-term of B cell ablation by cyclophosphamide or in BM chimeras.9,15,19 This failure, however, can be explained by the low frequency of lymphoid-biased HSCs in the old BM used in these experiments, as opposed to that found in the BM of young mice.24,40 Indeed, our finding that peripheral B-cell reconstitution in old mice after one round of depletion lasted more than 50 days is in agreement with these studies. However, we propose here that the chronic B-cell deficiency, obtained after one round of B-cell depletion (this study) or after BM or HSCs transfer into irradiated mice or in cyclophosphamide-treated mice,9,15,19 reactivates B lymphopoiesis in the BM, but long-term is necessary to allow progenitor (MPP and CLP) and precursor expansion to restore it into full capacity and to measurably detect it. In agreement with this, we show here that the rate of B-cell reconstitution in old mice increases after the second and the third rounds of depletion, reflecting the increase in frequencies of lymphoid-biased HSCs and progenitors on the peripheral demand. This observation may not have been detected in the previous studies9,15,19 because peripheral B-cell reconstitution was followed right after induction of the peripheral demand but not after repeated or long-term deficiency as we did here.
An alternative possibility for reactivation of B lymphopoiesis is that HSCs are capable to clear the accumulated defects to reverse cellular senescence. There are studies proposing that environmental factors17 and epigenetic modifications41 affect the entrance into cellular senescence. Because changes introduced by environment or by epigenetic mechanisms are reversible, it has been thought that cellular senescence can be reversed. Indeed, this was demonstrated in a study in which young and old mice shared their circulatory system by establishing parabiotic pairings. This study demonstrated that exposing aged skeletal stem cells to factors present in young serum restored their proliferation and regenerative capacity.42
Enhancing the immune competence to mount antibody responses to new antigens is of major importance to the elderly population.11-14 We show here that mice treated for B-cell depletion developed significantly increased antibody responses to the NP-CGG challenge. The interpretation of this finding is that the peripheral compartment that is reconstructed in old mice after B-cell depletion is more competent in recognition and responsiveness to new antigenic challenge. Yet, the antibody responses mounted by the rejuvenated repertoire were still lower compared with the responses developed in young mice. This may reflect age-related alterations in T cells and in innate immune cells,3,43 which have not been replaced in these mice, perhaps contributing to the incomplete restoration of immune responsiveness. Similarly, rheumatoid arthritis patients treated with B-cell depletion therapy generate a diverse repertoire of B cells that is mainly derived from newly generated B cells in the BM.44,45 In old mice, the rejuvenated repertoire produced a significant increase in antibodies to a new antigenic challenge. It will be interesting to find whether old patients treated for B-cell depletion can also mount an increased antibody response to new antigens.
In conclusion, an important question that arises here is how chronic B-cell deficiency is transcribed into a sensing mechanism of feedback regulation allowing a cross-talk between the peripheral and the progenitor B-cell compartments. It is tempting to speculate that a soluble factor, which is produced in the periphery (perhaps by long-lived B cells), has an inhibition/activation capacity on B lymphopoiesis in the BM (such as erythropoietin in the homeostasis of red blood cells36 ). The observation that MPP and CLP populations are also expanding on B-cell depletion suggests that the sensing mechanism operates at a high hierarchy of the hematopoietic system to increase proliferation and/or commitment to the B lineage. The changes in BM microenvironment and their effect on the alteration of hematopoiesis and lineage potential of stem cells with aging support this possibility (reviewed by Wagner et al46 ). B-cell depletion is also associated with reduction of serum immunoglobulins,28 but this factor appears not to affect B-cell generation in the BM.47-49 It is also possible that competition on niche space in the BM between developing, mature, and plasma cells plays a role in regulation of B-cell development.50 Studies to understand how this cross-talk is mediated are now conducted.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Klaus Rajewsky for providing the BAFF-RFL/FL Mx-cre mice, Dr Frank Costantini for providing the R26R-EYFP mice, Dr Marc Shlomchik for providing the hCD20 Tg mice, and Dr Ramit Mehr for thoughtful discussions and critical reading of the manuscript.
This work was supported by the Israel Science Foundation, the Wolens Gerontology Research Fund, the Weisz Gerontology Research Fund, and the Atkins Medical Research Fund.
Authorship
Contribution: Z.K., S. Naor, and S. Nussbaum designed and performed the research, analyzed the data, and wrote the manuscript; K.G., T.I., and T.L. preformed experiments and analyzed data; Y.S. and M.S.-S. contributed a new analytic tool; and D.M. was the principal investigator in this study, designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Doron Melamed, Technion, Faculty of Medicine, Department of Immunology, Haifa 31096, Israel; e-mail: melamedd@tx.technion.ac.il.
References
Author notes
Z.K. and S. Naor contributed equally to this study

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal