Abstract
The early emergence of macrophages and their large pattern of tissue distribution during development suggest that they may play a critical role in the initial steps of embryogenesis. In the present study, we show that monocytic cells derived from human embryonic stem cells (hESCs) and from fetal liver follow a differentiation pathway different to that of adult cells, leading to specific functions. Embryonic and fetal monocytic cells differentiated from a CD14lowCD16− precursor to form CD14highCD16+ cells without producing the CD14highCD16− cell population that predominates in adult peripheral blood. Both demonstrated an enhanced expression of genes encoding tissue-degrading enzymes, chemokines, and scavenger receptors, as was previously reported for M2 macrophages. Compared with adult blood monocytes, embryonic and fetal monocytic cells secreted high amounts of proteins acting on tissue remodeling and angiogenesis, and most of them expressed the Tie2 receptor. Furthermore, they promoted vascular remodeling in xenotransplanted human tumors. These findings suggest that the regulation of human fetal and embryonic monocytic cell differentiation leads to the generation of cells endowed mainly with anti-inflammatory and remodeling functions. Trophic and immunosuppressive functions of M2-polarized macrophages link fetus and tumor development, and hESCs offer a valuable experimental model for in vitro studies of mechanisms sustaining these processes.
Introduction
The mononuclear phagocyte system (MPS) is a network of highly versatile and multifunctional cells that include peripheral blood monocytes, dendritic cells, and tissue macrophages, which play major roles in development, scavenging, inflammation, and antipathogen defenses.1-3 These cells originate from hematopoietic stem cells in the bone marrow through differentiation steps generating common myeloid progenitors shared with neutrophils, then enter the blood as monocytes to be recruited into normal healthy tissues or at sites of injury, where they differentiate into dendritic cells or macrophages.1,4 Macrophages demonstrate a remarkable heterogeneity related to their phenotype, localization, and function.1,3,5
It has been proposed to distinguish between M1 macrophages attributed with proinflammatory functions and M2 macrophages attributed with wound-healing or tissue-remodeling functions, which is an overly simplistic but useful categorization of this complex cell lineage.6,7 It remains unsolved whether these heterogeneous functions are determined only by the local tissue environment or if they also reflect specific developmental maturation pathways.5,8,9
In mice and birds, macrophages are part of primitive hematopoiesis and first emerge in the yolk sac at day 7.5 postcoitum. A second wave of macrophages is formed in the yolk sac between days 8 and 8.5 postcoitum through maturation of a myeloid-erythroid progenitor. These macrophages resemble those appearing later from the differentiation of a lympho-myeloid hematopoietic stem cell present in the fetal liver.10,11 Macrophages generated in the fetal liver are rapidly dispersed in all embryonic tissues.12 The early emergence of macrophages and their large pattern of tissue expression during development suggest a critical role of these cells in the initial steps of embryogenesis.13,14
As is the case in adult mice, macrophages play a key role in the innate response to pathogens and constitute the primary host defense in the mouse embryo. Several lines of evidence also suggest trophic roles for mouse embryonic macrophages, including bone morphogenesis, ductal branching, neuronal networking, and angiogenesis.14,15 The gene signature of mouse embryonic macrophages is shared with a subset of adult peripheral blood monocytes expressing the angiopoietin receptor Tie2, known as Tie-2–expressing monocytes (TEMs), which may be part of the group of Gr1−/Ly-6clo/CX3CR1high/CCR2−/CD62L− cells.16,17 Their human counterparts belong to the less-frequent subpopulation of “nonclassic” circulating monocytes with a CD14+CD16+ phenotype.3,18 Part of the embryonic mouse macrophage signature is shared with the so-called tumor-associated macrophages, which have tumor-remodeling and immunosuppressive functions.13,17,19-21 These data suggest that embryonic mouse macrophages are specialized in tissue remodeling and that similar cells are recruited or reprogrammed during tumor development.14 Common remodeling and immunosuppressive functions of M2-polarized macrophages may link normal developmental and pathologic processes.
Our knowledge on the development and functional properties of the human MPS during ontogeny is much less extensive because of the limited availability of human embryos at the earliest stages of development.22 In recent years, human embryonic stem cells (hESCs) have been used as an in vitro model of the early stages of tissue maturation. In vitro hESC hematopoietic differentiation recapitulates the main stages of early in vivo embryogenesis,23-27 so this model was used in the present study for studying embryonic monopoiesis. More specifically, we examined monocytes/macrophages obtained from differentiation of hESCs and fetal liver CD34+ cells issued from first-trimester fetuses. We show that, in the early stages of human development, most of the generated fetal and embryonic monocytic cells share a specific pathway of differentiation that is correlated with transcriptional and functional patterns that characterize anti-inflammatory and tissue-remodeling cells.
Methods
hESC maintenance and differentiation
H1 (NIH code WA01) and H9 (NIH code WA09) hESC cell lines were obtained from the WiCell Research Institute. Most experiments were performed with the H9 cell line. Undifferentiated hESCs were grown as described previously. Differentiation was achieved from embryoid bodies (EBs), and the differentiation medium consisted of Iscove modified Dulbecco medium (IMDM) supplemented with 15% fetal bovine serum (FBS). Mesoderm and hematopoietic specifications were induced by adding bone morphogenetic protein 4 (BMP-4, 10 ng/mL), vascular endothelial growth factor (VEGF, 5 ng/mL), interleukin-3 (IL-3, 100 U/mL), and fetal liver tyrosine kinase 3 ligand (FLT3-L, 10 ng/mL; all from Peprotech); stem cell factor (SCF, 50 ng/mL; a gift from Amgen, Thousand Oaks, CA); and thrombopoietin (TPO, 10 ng/mL; a gift from Kirin Laboratories, Tokyo, Japan). CD14+ cells were sorted and their terminal differentiation toward the monocyte/macrophage lineage was achieved in presence of monocyte-colony–stimulating factor (M-CSF, 50 ng/mL), granulocyte-macrophage colony–stimulating factor (GM-CSF, 20 ng/mL), IL-3 (100 U/mL), and FLT3-L (10 ng/mL; Peprotech). Cultures were maintained in a 5% CO2/5% O2 environment and the differentiation medium was changed every 4 days.
Generation of monocytes/macrophages from fetal and adult CD34+ cells
Adult CD34+ cells were recovered from cytapheresis performed in healthy donors submitted to hematopoietic stem cell harvest. Fetal CD34+ cells were recovered from fetal liver ranging from 10-17 weeks estimated gestational age, which were obtained after legal abortion. CD34+ cells from fetal liver or from adult peripheral blood mononuclear cells (PBMCs) were purified using CD34 immunomagnetic beads (MACS; Miltenyi Biotec). Proliferation and differentiation of CD34+ cells toward the monocyte/macrophage lineage was achieved in conditions similar to those described for hESCs in the presence of M-CSF, GM-CSF, IL-3, and FLT3-L.
Human PBMC sorting
PBMCs were sorted from cytaphereses performed in healthy donors, isolated using a Ficoll gradient, and monocytes were enriched by adherence in IMDM supplemented with 15% FBS.
Flow cytometry and cell sorting
To obtain a single-cell suspension, EB cultures were treated with trypsin-EDTA (trypsin-ethylenediaminetetraacetic acid, 0.25%; Sigma-Aldrich) for 5 minutes at 37°C. Adherent monocytes from hESCs, fetal liver, and blood CD34+ cells or from the peripheral blood were detached from the plastic plates by incubation with cold EDTA/phosphate-buffered saline (2mM) for 10 minutes. The single-cell suspension was incubated for 30 minutes at 4°C with the different conjugated monoclonal antibodies (MoAbs) and washed twice. Flow cytometric characterization was performed using a panel of anti–human MoAbs: phycoerythrin (PE)–conjugated anti-CD14, -CD16, -CD163, -CD64, -CCR5, and -CD34 (all from Becton Dickinson); Tie2 (R&D Systems); fluorescein isothiocyanate (FITC)–conjugated anti-CD16 and -CD15 (Becton Dickinson); allophycocyanin (APC)–conjugated CD14, CD32, CD15, CD36, and CD62L (Becton Dickinson); CD115 (R&D Systems); and CD16 (Caltag Laboratories). Cells were analyzed with a FACSort flow cytometer (Becton Dickinson). The percentage of positive cells was determined by comparison with isotypic controls, establishing the background level of nonspecific staining. CellQuest software Version 4.0 (Becton Dickinson) was used for data acquisition and analysis. CD14+ or CD14+CD16− and CD14+CD16+ cells were sorted after labeling with PE-conjugated anti-CD14 and APC-conjugated anti-CD16 MoAbs using a FACSDiva cell sorter (Becton Dickinson).
Cell morphology
CD14+ cells obtained from hESCs were sorted after 21 days of differentiation from EB cultures and then cultured in the presence of FLT3-L, IL-3, GM-CSF, and M-CSF. Differentiated cells were stained with May-Grünwald-Giemsa solution, myeloperoxidase, and the double-specific (naphthol AS-D chloroacetate esterase)/nonspecific (-naphthyl butyrate esterase) esterase staining kit (Sigma-Aldrich) in accordance with the manufacturer's protocols.
Quantification of hematopoietic progenitors in semisolid cultures
To study hematopoietic progenitors, dissociated cells from EBs were plated in triplicate at a density of 3 × 104 cells/mL in human methylcellulose medium H4434 containing cytokines (StemCell Technologies). Progenitor-derived hematopoietic colonies were scored after 9-14 days.
Total RNA isolation, RT-PCR, and quantitative RT-PCR
CD14+CD16+ cells were sorted from embryonic or fetal cells or from adult PBMCs (> 4 repeated experiments in each condition). When indicated, cells were activated with 100 ng/mL of lipopolysaccharide (LPS; Sigma-Aldrich) and 1000 U/mL of interferon-γ (IFN-γ; PeproTech) for 24 hours. Total RNA was isolated using the RNeasy Micro Kit (QIAGEN), and the RNA concentration was adjusted to 100 ng/μL (NanoDrop Technologies). Quality was assessed using the Lab-on-a-Chip 2100 Bioanalyzer technology (Agilent Technologies). For each condition, the RNA of 3 experiments was mixed, a total of 1 μg of RNA was reverse transcribed into cDNA with SuperScript III (Invitrogen), and quantitative RT-PCR analyses were performed with TaqMan low-density arrays. cDNA (0.1-1 μg) was loaded on immune panel and customized gene array (Applied Biosystems). Quantitative RT-PCR was run for 35 cycles in standard mode using an ABI7900HT apparatus (Applied Biosystems). Raw data were extracted using SDS 2.2.1 software, and the difference between the threshold cycle (CT) of each gene and that of the endogenous controls 18S and GAPDH was used to determine (ΔCT) gene expression.
Cytokine and chemokine secretion and expression
CD14+CD16+-sorted cells (300 × 103) were washed and cultured for 24 hours in 0.2 mL of IMDM with 10% FBS. Cells were either untreated or stimulated with LPS or IFN-γ (n = 4 repeated experiments in each condition). Levels of 20 cytokines released into the supernatants were determined using a customized Procarta Cytokine Assay Kit (Ozyme) and xMAP Technology multianalyte profiling (Luminex). Quantification of cytokines was realized using the Bio-Plex 200 (Bio-Rad). The culture medium was used as a negative control. Data were analyzed with the BioManager 5.0 software (BioRad) using 4-5 parameter values. The Human Angiogenesis Array Kit (R&D Systems) was used to measure the relative levels of tissue remodeling–related proteins (n = 55) following the Proteome Profiler Array technology. All experiments were performed in duplicate.
In vivo tumor angiogenesis assays
All animal treatment and procedures were approved by the Institut Gustave Roussy animal care and use committee. Sorted CD14+CD16+ from hESCs, fetal CD34+ cultures, and adult peripheral blood cells (2.5 × 105) were coinjected subcutaneously with U87 human glioma cells (5 × 106) in nude mice; control mice received only glioma cells (n = 6 for each group). Tumors were grown for 5-7 days. To quantify angiogenesis, serial sections spanning the whole tumor were immunostained for CD34 (anti–mouse CD34; Hycult Biotechnology), followed by incubation with a rabbit antibody (SouthernBiotech) for 1 hour. Quantification of CD34-stained microvessels was achieved using PIXCIT, a software package designed by the Groupe Régional d'Etudes sur le Cancer (Caen, France). Cell quantification was also performed using flow cytometric analysis with a PE-conjugated anti–human CD45 MoAb and an FITC-conjugated anti–mouse CD34 MoAb (n = 6 for each group).
Phagocytosis assay
Cells (250 000) were suspended in 0.5 mL of Hank buffered salt solution containing 10μM luminol, incubated at 37°C in a thermostatic luminometer, and stimulated (or not) with opsonized zymosan (0.5 mg/mL; Sigma-Aldrich). Changes in chemiluminescence were measured over a 30-minute period.
Statistical analysis
Differences in quantitative RT-PCR and protein analysis and in tumor angiogenesis assays between groups were assessed by pairwise t tests (Software Inc). The pooled standard deviation (SD) switch calculated a common SD for all groups and was used for all comparisons. The reported P value is a result of the Bonferroni adjustment method. P < 5% was considered statistically significant.
Results
Embryonic monocytes and macrophages develop from hESCs through a process similar to bone marrow monopoiesis
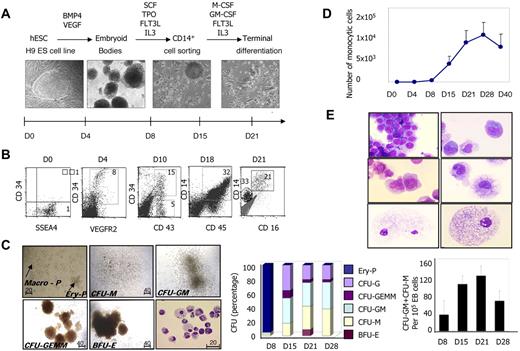
We induced human ESC differentiation into the monocyte/macrophage lineage through a 3-step protocol (Figure 1A). Step 1 was EB formation by culturing the SSEA4+ H9 cell line (Figure 1B) in low-attachment plates in the presence of BMP-4 and VEGFA for 5 days, which led to mesoderm specification attested by the appearance of CD34+VEGFR2+ cells (Figure 1B). Step 2 was hematopoietic differentiation in the presence of SCF, FLT3-L, TPO, and IL-3 from days 5-21, which resulted in the emergence of CD34+CD43+ cells (Figure 1B), a population of embryonic hematopoietic progenitors. Hematopoietic cells were detected in EBs and floating cells emerging from EBs, and included cells expressing monocytic lineage markers, CD45, CD14 (Figure 1B), and CD115 (M-CSFR, shown subsequently) as early as day 14. Step 3 was the expansion and differentiation of the monocyte/macrophage lineage by sorting CD14+ cells between days 14 and 21, and then culturing these cells in the presence of M-CSF, GM-CSF, FLT3-L, and IL-3, which efficiently supported the development of adherent cells with typical macrophage morphology (Figure 1A-B).
Differentiation of hESCs into monocytes/macrophages. (A) Description of the 3-step protocol designed to induce hESC differentiation into monocyte/macrophages: step 1: EB formation with mesoderm specification in the presence of BMP-4 and VEGFA; step 2: hematopoiesis induction in the presence of SCF, TPO, FLT3-L, and IL-3; and step 3: culture of CD14+ cells sorted between days 14 and 21 in the presence of M-CSF, GM-CSF, FLT3-L, and IL-3. (B) hESC-derived cells were characterized by cell surface marker analysis using flow cytometry at the indicated times of the culture. (C) Methylcellulose-based colony-forming assay: (→) typical primitive erythroid (Ery-P) and macrophage (Macro-P) colonies and definitive (BFU-E, CFU-G, CFU-GM, CFU-M, and CFU-GEMM) colonies. Original magnification was 20× (Ery-P and Macro-P) and 10× (definitive colonies). Cells were assessed for their ability to form erythroid and myeloid colonies in a standard methylcellulose assay at the indicated times of the culture. The middle panel shows the percentages of the indicated colonies. The right panel shows the absolute numbers of CFU-GM + CFU-M per 105 total EB cells. Results are mean ± SD of 3 independent time-course experiments with methylcellulose assays performed in triplicate and colonies enumerated at days 9-10. (D) Proliferative potential of monocytic cells; the percentage of CD14+ cells was measured at different times during the culture and related to the total number of cells, including adherent, floating, and those from EB cells. (E) CD14+ cells from step 2 were sorted, cultured, harvested at different times, and stained with May-Grünwald-Giemsa solution. Typical morphologic features of monoblasts (top panel), monocytes (middle panel), and macrophages (bottom panel) are shown. Photographs were taken with a 63× objective.
Differentiation of hESCs into monocytes/macrophages. (A) Description of the 3-step protocol designed to induce hESC differentiation into monocyte/macrophages: step 1: EB formation with mesoderm specification in the presence of BMP-4 and VEGFA; step 2: hematopoiesis induction in the presence of SCF, TPO, FLT3-L, and IL-3; and step 3: culture of CD14+ cells sorted between days 14 and 21 in the presence of M-CSF, GM-CSF, FLT3-L, and IL-3. (B) hESC-derived cells were characterized by cell surface marker analysis using flow cytometry at the indicated times of the culture. (C) Methylcellulose-based colony-forming assay: (→) typical primitive erythroid (Ery-P) and macrophage (Macro-P) colonies and definitive (BFU-E, CFU-G, CFU-GM, CFU-M, and CFU-GEMM) colonies. Original magnification was 20× (Ery-P and Macro-P) and 10× (definitive colonies). Cells were assessed for their ability to form erythroid and myeloid colonies in a standard methylcellulose assay at the indicated times of the culture. The middle panel shows the percentages of the indicated colonies. The right panel shows the absolute numbers of CFU-GM + CFU-M per 105 total EB cells. Results are mean ± SD of 3 independent time-course experiments with methylcellulose assays performed in triplicate and colonies enumerated at days 9-10. (D) Proliferative potential of monocytic cells; the percentage of CD14+ cells was measured at different times during the culture and related to the total number of cells, including adherent, floating, and those from EB cells. (E) CD14+ cells from step 2 were sorted, cultured, harvested at different times, and stained with May-Grünwald-Giemsa solution. Typical morphologic features of monoblasts (top panel), monocytes (middle panel), and macrophages (bottom panel) are shown. Photographs were taken with a 63× objective.
Using a methylcellulose-based colony-forming assay, we detected the emergence, at day 8, of primitive erythroid colonies comprising 6-20 cells (Ery-P) and primitive macrophage colonies (Macro-P) similar to those described in mouse hematopoiesis and ex vivo human hemangioblast culture (Figure 1C left panel). At day 15, hematopoietic activity was mainly granulocyte and macrophage progenitors (CFU-G, CFU-GM, and CFU-M), with only rare erythroid (BFU-E) and mixed (CFU-GEMM) progenitors (Figure 1C left and middle panels). The production of macrophage progenitors peaked at day 21 (Figure 1C right panel). Cytologic examination revealed the presence of mature macrophages in CFU-M–, CFU-GM–, and CFU-GEMM–derived colonies (Figure 1C left panel). These data suggested 2 waves in the in vitro development of the embryonic monocyte/macrophage lineage. The second wave generated a progenitor hierarchy close to that observed during fetal or adult hematopoiesis. The proliferative potential of monocytic cells issued from these embryonic progenitors is shown in Figure 1D.
To dissect more accurately the maturation of embryonic monocytes and macrophages, CD14+ cells from step 2 were sorted, cultured, harvested at different times, and stained with May-Grünwald-Giemsa solution. After 1 week in culture, we observed a large spectrum of cells belonging to the monocyte/macrophage lineage (Figure 1D), ranging from monoblasts (Figure 1D top panel) to mature macrophages (Figure 1D bottom panel), with the presence of monocytes (Figure 1D middle panel). Later, the cultures contained mainly macrophages, which were negative for myeloperoxidase and positive for nonspecific esterase (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Electron microscopy showed typical features of macrophages with cytoplasmic pseudopodia, highly developed vacuoles, and rough dark granules of various densities (supplemental Figure 1B). This experimental approach enabled the efficient generation of hESC-derived monocytes and macrophages (subsequently named monocytic cells) through the main stages of adult monopoiesis.
CD16 expression identifies 2 populations of CD14+ hESC–derived cells
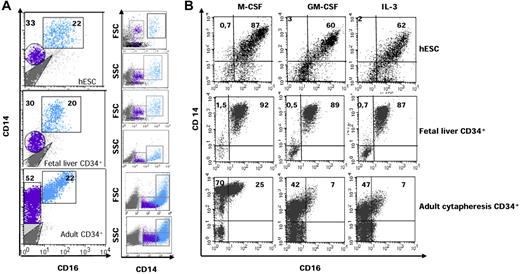
CD14 and CD16 (FcγR-III) are used to distinguish 2 subsets of monocytes with distinct functional properties in human adult peripheral blood.1,3,28 The major subset, called classic monocytes, expresses CD14 but lacks CD16 (CD14highCD16−), whereas a minor subset that expresses CD16 includes at least 2 populations with distinct functions, CD14highCD16+ and CD14lowCD16+ cells.29 Whatever their function, macrophages usually express both CD14 and CD16.30 Analysis of CD14 and CD16 expression at the surface of hESC-derived cells identified a major population of CD14highCD16+ cells and a minor population of CD14lowCD16− cells (Figure 2A top panel). These 2 subsets demonstrated distinct size and granularity, with CD14highCD16+ cells showing scatter properties similar to those of macrophages (Figure 2A top panel). This pattern of CD14 and CD16 expression was quite different from that reported for adult monocytes and macrophages. To determine whether this differential pattern was related to the ontogenic stage or to the experimental procedure, we cultured fetal liver and adult peripheral blood CD34+ cells according to step 3 of the culture protocol used for hESCs. Culture of fetal liver CD34+ cells for 10 days in the presence of M-CSF, GM-CSF, IL-3, and FLT3-L generated the same pattern of CD14 and CD16 expression as hESC-derived cells (Figure 2A middle panel), whereas adult CD34+ cells gave rise to CD14+CD16− cells with a large spectrum of CD14 expression (Figure 2A bottom panel); the latter included a majority of CD14highCD16− cells with low scatter properties, similar to peripheral blood classic monocytes. These results suggested that mononuclear phagocytes generated in the embryo and fetus might differ from those generated in adults.
Differential expression of CD14 and CD16 allows the characterization of 2 subsets of hESC- and fetal liver–derived CD14+ cells. (A) CD14 and CD16 expression on cells issued from day 21 hESCs (top panel), day 10 fetal liver CD34+ cells (middle panel), and day 10 adult peripheral blood CD34+ cells (bottom panel). hESCs were cultured as described in the legend to Figure 1; fetal liver and adult CD34+ cells were cultured in the presence of M-CSF (50 ng/mL), GM-CSF (20 ng/mL), IL-3 (100 U/mL), and FLT3-L (10 ng/mL). Blue indicates CD14highCD16+ cells; purple indicates CD14lowCD16− cells. Right panel shows the forward (FSC) and size (SSC) scatter properties of CD14+ cells. (B) Embryonic, fetal, and adult CD14lowCD16− cells were sorted and cultured in the presence of IL-3 or GM-CSF or M-CSF for 9 days before assessing cell surface expression of CD14 and CD16.
Differential expression of CD14 and CD16 allows the characterization of 2 subsets of hESC- and fetal liver–derived CD14+ cells. (A) CD14 and CD16 expression on cells issued from day 21 hESCs (top panel), day 10 fetal liver CD34+ cells (middle panel), and day 10 adult peripheral blood CD34+ cells (bottom panel). hESCs were cultured as described in the legend to Figure 1; fetal liver and adult CD34+ cells were cultured in the presence of M-CSF (50 ng/mL), GM-CSF (20 ng/mL), IL-3 (100 U/mL), and FLT3-L (10 ng/mL). Blue indicates CD14highCD16+ cells; purple indicates CD14lowCD16− cells. Right panel shows the forward (FSC) and size (SSC) scatter properties of CD14+ cells. (B) Embryonic, fetal, and adult CD14lowCD16− cells were sorted and cultured in the presence of IL-3 or GM-CSF or M-CSF for 9 days before assessing cell surface expression of CD14 and CD16.
We then sorted CD14lowCD16− cells and cultured these cells in the presence of IL-3 or GM-CSF or M-CSF. CD14lowCD16− cells derived from hESCs and fetal liver CD34+ cells rapidly formed groups of 4-10 round, proliferating cells that became larger clusters (10-50 cells) and semiadherent colonies (> 50 cells) after 7-10 days of culture (supplemental Figure 2A). At day 9, cells with morphologic characteristics of macrophages and a unique CD14highCD16+ phenotype were observed, whatever the cytokine conditions (Figure 2B top and middle panel). In contrast, sorted CD14lowCD16− cells derived from adult CD34+ cells gave rise to CD14highCD16− cells that subsequently differentiated into CD14highCD16+ cells (Figure 2B bottom panel). We subsequently focused on the phenotypic, molecular, and functional characterization of the embryonic and fetal CD14highCD16+ subset.
hESC- and fetal liver–derived monocytic cells demonstrate a nonclassic monocyte phenotype
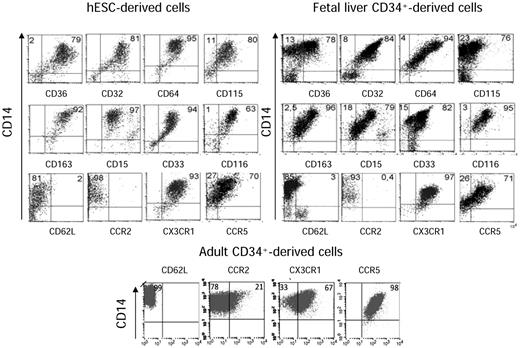
Additional immunophenotyping of the embryonic and fetal CD14highCD16+ subset revealed the expression of the scavenger receptors CD36 and CD163 and the Fc receptors CD32 (FcγRII) and CD64 (FcγRI). These cells also expressed CD15, CD33, and the cytokine receptors CD115 (M-CSFR) and CD116 (GM-CSFR). The majority of the cells expressed CCR5 and CX3CR1, but only marginal amounts of CD62L (L-selectin) and CCR2 (Figure 3 top panel). Monocytic cells generated from adult CD34+ cells under the same conditions expressed more CCR2 and CCR5 and less CX3CR1 compared with embryonic and fetal CD14highCD16+ (Figure 3 bottom panel). Expression of CX3CR1, CD115, and CD135 (FLT3) has been also described at the surface of the mouse macrophage-dendritic precursor31 ; we confirmed that hESC- and fetal liver–derived monocytic cells gave rise to dendritic cells after 8-10 days in the presence of GM-CSF and IL-4 (supplemental Figure 2B-C). These results reinforced the previous suggestion that embryonic and fetal monocytic cells are developmentally close and distinct from adult cells. We therefore determined the functional state of this population in reference to the subset of peripheral blood monocytes expressing the same CD14+16+ phenotype.
hESC- and fetal liver–derived CD14+CD16+ cells demonstrate a nonclassic monocyte phenotype. Immunophenotype analysis of hESC-derived (top left panel), CD34+ fetal liver–derived (top right panel), and CD34+ adult-derived (bottom panel) CD14+CD16+ cells. The percentages of cells expressing the indicated markers within CD14+ cells are shown. The majority of embryonic and fetal subsets expressed CCR5 and CX3CR1. When monocytic cells were generated from adult CD34+ cells, a marker of the inflammatory type, CCR2, was detected on a subset of cells. Gating was set according to the negative isotype controls. One of at least 3 representative experiments is shown. The corresponding cell frequencies as determined in 3 independent experiments are presented.
hESC- and fetal liver–derived CD14+CD16+ cells demonstrate a nonclassic monocyte phenotype. Immunophenotype analysis of hESC-derived (top left panel), CD34+ fetal liver–derived (top right panel), and CD34+ adult-derived (bottom panel) CD14+CD16+ cells. The percentages of cells expressing the indicated markers within CD14+ cells are shown. The majority of embryonic and fetal subsets expressed CCR5 and CX3CR1. When monocytic cells were generated from adult CD34+ cells, a marker of the inflammatory type, CCR2, was detected on a subset of cells. Gating was set according to the negative isotype controls. One of at least 3 representative experiments is shown. The corresponding cell frequencies as determined in 3 independent experiments are presented.
Cytokine expression in hESC- and fetal liver–derived monocytic cells evokes anti-inflammatory cells
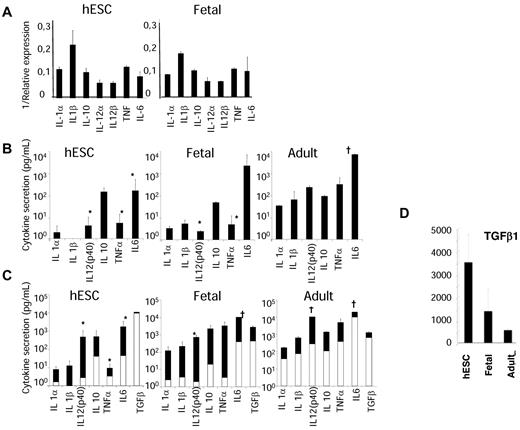
Cytokine production is the hallmark of the M1 versus M2 polarization paradigm in macrophages. hESC- and fetal liver–derived CD14highCD16+ cells demonstrated a similar pattern of immune and anti-inflammatory cytokine mRNA expression (supplemental Table 1). In both cases, IL-10 mRNA expression was higher than that of IL-12α and IL-12β mRNA, which suggested an M2 anti-inflammatory state (Figure 4A). These data were confirmed at the level of secreted proteins (Figure 4B). In contrast, CD14+CD16+ adult monocytes demonstrated a similar production of IL-10 and IL-12. Treatment of hESC- and fetal liver–derived monocytic cells with IFN-γ and LPS stimulated the production of proinflammatory cytokines. This production remained ∼10-fold lower than in their adult counterparts, with a significant differences in IL-12, IL-6, and TNFα secretion (P < .05; Figure 4C). Overall, levels of secreted cytokines in embryonic and fetal monocytic cells remained low compared with adult CD14+CD16+ monocytes, except for transforming growth factor-β (TGF-β), with a level 7- and 2.5-fold increased in the supernatants of embryonic and fetal cells, respectively (Figure 4D). These results suggested that hESC- and fetal liver–derived monocytic cells might have anti-inflammatory and immunosuppressive properties as well as tissue-remodeling activity.
hESC- and fetal liver–derived monocytic cells have a pattern of cytokine expression characteristic of an anti-inflammatory state. (A) Gene expression profile of sorted CD14+CD16+ from hESC-derived (n = 6) and fetal liver–derived (n = 4) CD34+ cell cultures. quantitative RT-PCR analysis shows the expression level of relevant genes of the M1 and M2 polarization paradigm. Results are expressed as 1/ΔCT values (mean ± SEM) over the endogenous controls 18S and Gadph. (B-C) Cytokine production was measured in the supernatant from 3 × 105 hESCs and fetal-sorted CD14+CD16+ cells, and comparatively assessed with CD14+CD16+ peripheral blood monocytes (n = 4 for each population). Tested cells were either left untreated (basal; B) or were stimulated with LPS and IFN-γ for 24 hours (C); the resulting supernatants were subsequently analyzed using a Luminex assay. Results are expressed in picograms per milliliter. Note that the response to LPS and IFN-γ in terms of inflammatory cytokine production is decreased from embryonic and fetal cells compared with the adult. (D) Comparative analysis of TGF-β secretion from hESC-derived, fetal liver–derived, and adult peripheral blood CD14+CD16+ cells. Note that in contrast to other cytokines, TGF-β was oversecreted by embryonic and fetal cells compared with adult cells. Results are expressed in picograms per milliliter. †Cytokine level was over the maximal range of the assay. Statistical differences between groups were calculated by pairwise t test. *P < .05 between the expression and production of some proinflammatory cytokines after stimulation with IFN-γ and LPS by adult versus hESC- and fetal liver–derived cells.
hESC- and fetal liver–derived monocytic cells have a pattern of cytokine expression characteristic of an anti-inflammatory state. (A) Gene expression profile of sorted CD14+CD16+ from hESC-derived (n = 6) and fetal liver–derived (n = 4) CD34+ cell cultures. quantitative RT-PCR analysis shows the expression level of relevant genes of the M1 and M2 polarization paradigm. Results are expressed as 1/ΔCT values (mean ± SEM) over the endogenous controls 18S and Gadph. (B-C) Cytokine production was measured in the supernatant from 3 × 105 hESCs and fetal-sorted CD14+CD16+ cells, and comparatively assessed with CD14+CD16+ peripheral blood monocytes (n = 4 for each population). Tested cells were either left untreated (basal; B) or were stimulated with LPS and IFN-γ for 24 hours (C); the resulting supernatants were subsequently analyzed using a Luminex assay. Results are expressed in picograms per milliliter. Note that the response to LPS and IFN-γ in terms of inflammatory cytokine production is decreased from embryonic and fetal cells compared with the adult. (D) Comparative analysis of TGF-β secretion from hESC-derived, fetal liver–derived, and adult peripheral blood CD14+CD16+ cells. Note that in contrast to other cytokines, TGF-β was oversecreted by embryonic and fetal cells compared with adult cells. Results are expressed in picograms per milliliter. †Cytokine level was over the maximal range of the assay. Statistical differences between groups were calculated by pairwise t test. *P < .05 between the expression and production of some proinflammatory cytokines after stimulation with IFN-γ and LPS by adult versus hESC- and fetal liver–derived cells.
Monocytic cells derived from hESCs and fetal liver have a gene signature of tissue-remodeling activity
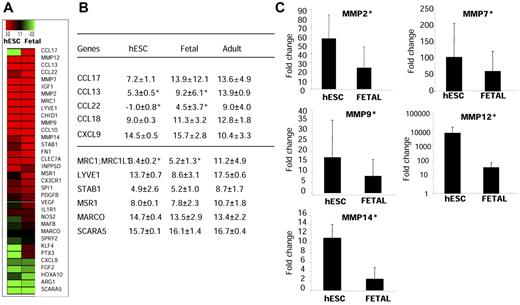
We used quantitative RT-PCR to compare the expression of 48 genes with products involved in tissue remodeling, angiogenesis, immune response, scavenging, and chemotaxis in CD14+CD16+ cells derived from hESCs, those derived from fetal liver CD34+ cells, or those isolated from adult peripheral blood. The expression of these genes was similar in hESC- and fetal liver–derived monocytic cells compared with that of adult CD14+CD16+ peripheral blood monocytes (Figure 5A). By pair-wise comparisons using the t test, we observed a significant increase in the expression of genes encoding chemokines (CCL22, CCL17, and CCL13; P = .01) related to an M2 polarization state, and a decrease in the expression of the gene encoding the Th1 chemokine CXCL9 (P = .05) in hESC- or fetal liver–derived cells compared with sorted adult cells (Figure 5A-B). hESC- and fetal liver–derived cells exhibited a several-fold up-regulation of genes encoding tissue-degrading enzymes such as metalloelastases and metalloproteases, including MMP2, MMP12, and MMP1 (P = .01) and MMP7 and MMP9, compared with adult monocytes (Figure 5A-B). hESC- and fetal liver–derived cells also expressed high levels of scavenger receptor–encoding genes MSR1 (macrophage scavenger receptor 1) and STABILIN-1, whereas the class A scavenger receptor gene SCARA5 was not detected. Compared with the adult subset, hESC- and fetal liver–derived cells expressed high levels of the phagocytic mannose receptor gene MRC1, MARCO, or the hyaluronan receptor-1 gene LYVE-1, which are characteristic markers of M2 macrophages (supplemental Table 2). This transcriptional pattern indicated that hESC- and fetal liver–derived monocytic cells have characteristic M2-polarized macrophages known to exert a wide range of homeostatic functions.
hESC- and fetal liver–derived monocytic cells share a distinguishing signature of tissue-remodeling activity. hESC- and fetal liver–derived CD14+CD16+ cells were sorted, and the expression of genes typical of M2 polarization were analyzed in comparison with adult blood sorted CD14+CD16+ cells using a customized gene array (Applied Biosystems; n = 4 for each population). (A) Heat-map representation of a common M2 gene signature shared by hESC- and fetal liver–derived monocyte/macrophages. (B) Relative mRNA expression (ΔCT) for receptor and chemokines tested. Expression of genes was normalized over the endogenous controls 18S and Gapdh; the higher the ΔCT, the lower the gene expression. (C) Expression of metalloproteases in hESCs and fetal cells is shown in terms of amplification versus expression in adult cells. Embryonic and fetal macrophages exhibited a several-fold up-regulation of several metalloproteases (MMP2, MMP7, MMP9, MMP12, and MMP14). *Increased expression of genes encoding metalloproteases and chemokines in hESC-derived cells compared with adult cells by pairwise comparisons using t test of genes (P = .01). A decreased expression of the Th1 chemokine CXCL9 was found in embryonic cells (P = .05).
hESC- and fetal liver–derived monocytic cells share a distinguishing signature of tissue-remodeling activity. hESC- and fetal liver–derived CD14+CD16+ cells were sorted, and the expression of genes typical of M2 polarization were analyzed in comparison with adult blood sorted CD14+CD16+ cells using a customized gene array (Applied Biosystems; n = 4 for each population). (A) Heat-map representation of a common M2 gene signature shared by hESC- and fetal liver–derived monocyte/macrophages. (B) Relative mRNA expression (ΔCT) for receptor and chemokines tested. Expression of genes was normalized over the endogenous controls 18S and Gapdh; the higher the ΔCT, the lower the gene expression. (C) Expression of metalloproteases in hESCs and fetal cells is shown in terms of amplification versus expression in adult cells. Embryonic and fetal macrophages exhibited a several-fold up-regulation of several metalloproteases (MMP2, MMP7, MMP9, MMP12, and MMP14). *Increased expression of genes encoding metalloproteases and chemokines in hESC-derived cells compared with adult cells by pairwise comparisons using t test of genes (P = .01). A decreased expression of the Th1 chemokine CXCL9 was found in embryonic cells (P = .05).
Monocytic cells derived from hESCs and fetal liver express tissue-remodeling proteins
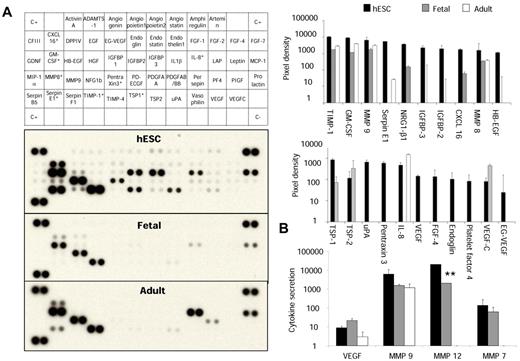
We used a protein array to explore the expression of 55 proteins involved in angiogenesis, vascular morphogenesis, and tissue remodeling in hESC- and fetal liver–derived monocytic cells compared with sorted adult CD14+CD16+ cells. Embryonic macrophages extensively secreted proteins acting on extracellular matrix and tissue remodeling, such as TIMP-1, MMP-8, and Serpins (Figure 6A). Analysis of secreted protein using Luminex technology corroborated these data and identified an impressive secretion of MMP-12 several times higher than in adult cultures, which is in agreement with transcriptional studies (P = .04; Figure 6B). Surprisingly, the secretion pattern of the fetal subset was closer to adult than to embryonic macrophages (Figure 6A and supplemental Table 3). Proteins acting specifically on angiogenesis, including VEGF, angiopoietin, thrombospondin, urokinase plasminogen activator, and pentraxin 3, were secreted at a lower level (Figure 6B), but embryonic macrophages still secreted a larger amount of angiogenic proteins compared with adult blood CD14lowCD16+ monocytes; these included ∼ 70% TEMs known for their pro-angiogenic activity.18 Proteins significantly increased in hESC-derived cells compared with those in fetal and adult cells are shown in Figure 6B. We confirmed that the specific profile of embryonic and fetal monocytic cells was not related to culture conditions by comparison with adult CD34+-derived monocytic cells obtained in the same culture conditions (n = 3; supplemental Figure 3).
Functional activity of hESC- and fetal liver–derived monocytic cells. (A) Protein arrays analysis in culture supernatants of hESC-derived, fetal liver–derived, and peripheral blood CD14+CD16+ cells. Array signals from scanned X-ray film images were analyzed using digital image analysis software. Array images from 10-minute exposure to X-ray film (left panel) and profiles created by quantifying the mean spot pixel densities (right panel) are shown. In the top right panel, note that the broadest pattern of expression associated with various proteins acting on extracellular matrix and tissue remodeling was found in the CD14highCD16+ embryonic cells. In the bottom right panel, the secretion of proteins involved in angiogenesis in the 3 populations is also shown. *Proteins significantly increased in hESC-derived cells compared with fetal and adult cells using pairwise comparisons with t test (P < .05). (B) Quantification of MMP secretion by Luminex technology. Note the secretion of large amounts of MMP-12 in embryonic cells compared with fetal and adult cells. **P = .04. Results are expressed in picograms per milliliter.
Functional activity of hESC- and fetal liver–derived monocytic cells. (A) Protein arrays analysis in culture supernatants of hESC-derived, fetal liver–derived, and peripheral blood CD14+CD16+ cells. Array signals from scanned X-ray film images were analyzed using digital image analysis software. Array images from 10-minute exposure to X-ray film (left panel) and profiles created by quantifying the mean spot pixel densities (right panel) are shown. In the top right panel, note that the broadest pattern of expression associated with various proteins acting on extracellular matrix and tissue remodeling was found in the CD14highCD16+ embryonic cells. In the bottom right panel, the secretion of proteins involved in angiogenesis in the 3 populations is also shown. *Proteins significantly increased in hESC-derived cells compared with fetal and adult cells using pairwise comparisons with t test (P < .05). (B) Quantification of MMP secretion by Luminex technology. Note the secretion of large amounts of MMP-12 in embryonic cells compared with fetal and adult cells. **P = .04. Results are expressed in picograms per milliliter.
Embryonic and fetal CD14highCD16+ cells demonstrated phagocytic functions that contributed to their remodeling activity. They showed the ability to ingest apoptotic cells, including embryonic erythrocytes and granulocytes (supplemental Figure 4A top left panel) and Escherichia coli at 37°C (supplemental Figure 4A top right panel). Phagocytosis of immunoglobulin G–opsonized E coli induced actin polymerization and the formation of a phagocytic cup in hESC-derived macrophages (Figure 7A bottom panel), and phagocytosis of opsonized zymosan particles triggered an oxidative burst as measured by chemiluminescence (supplemental Figure 4B).
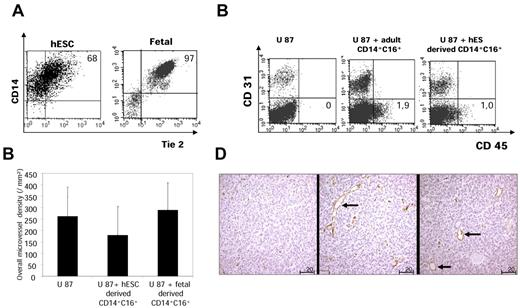
Angiogenic phenotype and function of hESC- and fetal liver–derived CD14+CD16+ monocytic cells. (A) Tie2 expression analysis. hESCs and fetal liver–derived CD14+CD16+ cells were costained with human Tie2 antibody, and expression was studied by cytometric analysis. Plots from one representative experience are shown. The percentage of Tie2-expressing cells is the mean value of 3 independent experiments. Tie-2 was expressed by the majority of embryonic and by virtually all fetal monocytes/macrophages. (B) Human glioma U87 cells were injected subcutaneously into nude mice alone (control) or with hESC- and fetal liver–derived CD14+CD16+ cells (n = 6 for each group). Developing CD34+ blood vessels were studied by immunochemistry at days 5-7. The vascular area was calculated by digital image analysis based on the quantification of mouse CD34–labeled vessels. Error bars indicate SD. Computer-assisted image analysis showed that the overall vascular area was not significantly greater in tumors coinjected with embryonic/fetal macrophages than in control tumors. (C) Detection of human cells by flow cytometric analysis. After administration of human glioma U87 cells into nude mice alone (control) or with hESC-derived and adult CD14+CD16+ cells (n = 6 for each group), at day 7 tumors were dissociated into single-cell suspensions and labeled with mouse CD31-PE and human CD45-APC. One representative experiment is shown. Results are mean values of the percentage of human CD45+ cells present in the tumors on the days of analysis (n = 6 for each group). *P < .05 for the statistical difference between the residual human CD45+ cells on the days of analysis after administration of adult and hESC-derived CD14+CD16+ cells. (D) Morphology of vessel sections in tumors coinjected with adult (left panel) or hESC CD14+CD16+ (middle and right) cells. Magnification is 200×. Note the larger vessel lumen after embryonic cell coadministration (→).
Angiogenic phenotype and function of hESC- and fetal liver–derived CD14+CD16+ monocytic cells. (A) Tie2 expression analysis. hESCs and fetal liver–derived CD14+CD16+ cells were costained with human Tie2 antibody, and expression was studied by cytometric analysis. Plots from one representative experience are shown. The percentage of Tie2-expressing cells is the mean value of 3 independent experiments. Tie-2 was expressed by the majority of embryonic and by virtually all fetal monocytes/macrophages. (B) Human glioma U87 cells were injected subcutaneously into nude mice alone (control) or with hESC- and fetal liver–derived CD14+CD16+ cells (n = 6 for each group). Developing CD34+ blood vessels were studied by immunochemistry at days 5-7. The vascular area was calculated by digital image analysis based on the quantification of mouse CD34–labeled vessels. Error bars indicate SD. Computer-assisted image analysis showed that the overall vascular area was not significantly greater in tumors coinjected with embryonic/fetal macrophages than in control tumors. (C) Detection of human cells by flow cytometric analysis. After administration of human glioma U87 cells into nude mice alone (control) or with hESC-derived and adult CD14+CD16+ cells (n = 6 for each group), at day 7 tumors were dissociated into single-cell suspensions and labeled with mouse CD31-PE and human CD45-APC. One representative experiment is shown. Results are mean values of the percentage of human CD45+ cells present in the tumors on the days of analysis (n = 6 for each group). *P < .05 for the statistical difference between the residual human CD45+ cells on the days of analysis after administration of adult and hESC-derived CD14+CD16+ cells. (D) Morphology of vessel sections in tumors coinjected with adult (left panel) or hESC CD14+CD16+ (middle and right) cells. Magnification is 200×. Note the larger vessel lumen after embryonic cell coadministration (→).
Monocytes/macrophages derived from hESCs and fetal liver promote tissue remodeling
The Tie-2 angiopoietin receptor is expressed on a subset of CD14+CD16+ adult peripheral blood monocytes, and Tie-2–expressing cells are required for the vascularization and growth of several tumor models.18 Tie-2 was detected at the surface of ∼ 70% of embryonic and virtually all fetal monocytes/macrophages (Figure 7A). Sorted hESC- and fetal liver–derived CD14highCD16+ cells were coinjected subcutaneously with human U87 glioma cells in nude mice, a xenogenic model used for the demonstration of the proangiogenic effect of adult TEMs. U87 cells alone or coinjected with adult CD14+CD16+ monocytes were used as controls. Developing CD34+ blood vessels were studied in tumors by immunochemistry at days 5-7. Computer-assisted image analysis showed that the overall vascular area was not significantly greater in tumors produced by coinjection of U87 cells with embryonic or fetal macrophages than in controls (Figure 7B). However, despite the early times of tumor growth (5-7 days), we observed a profuse vascular framework both in control and coinjected tumors (supplemental Figure 4). In an independent set of experiments, we observed, using cytometric analysis, that embryonic, fetal, and adult cells were still present in the tumors on the days of analysis, but at a significant larger extent for adult CD14+CD16+ monocytes (P < .05; Figure 7C). Additional detailed morphologic analysis showed that embryonic and fetal macrophages promoted the development of large blood vessels with wide lumens, whereas CD14+CD16+ adult monocytes induced a framework of small capillaries (Figure 7D). Thus, embryonic and fetal monocytes/macrophages promote vascular morphogenesis and tissue remodeling.
The specific pattern of CD14 and CD16 expression, inflammatory cytokines, and remodeling proteins was confirmed from monocytic cells issued from the H1 embryonic stem cell line (WiCell Research Institute; supplemental Figure 5).
Discussion
The ontogeny of monocyte/macrophage development has been investigated mainly in mice.10,12,14,32 Therefore, the establishment of MPS in humans remains poorly understood. The present study used hESC and fetal liver cell differentiation models to gain insights into the ontogeny of monocytes/macrophages in humans. Using these 2 independent experimental approaches, we demonstrated that both hESC- and fetal liver–derived monocytes/macrophages arise through a similar developmental process and are specialized for specific homeostatic functions.
Monocytes/macrophages have been previously derived from hESCs by 2 approaches. One used hESC differentiation into hematopoietic cells on OP9 cells and led to the identification of a GM progenitor that differentiated into monocytes/macrophages.33 The second one used EB formation followed by monocyte/macrophage differentiation in the presence of M-CSF and IL-3.34 In the present study, we set up a stepwise culture method with defined combinations of cytokines, which allowed us to generate functional mature monocytes and macrophages. We identified 2 sequential waves of embryonic monopoiesis temporally linked with the emergence of primitive and definitive erythroid colonies, suggesting that this experimental protocol reproduces the main stages of embryonic hematopoiesis. Clonogenic assays and cytologic examination confirmed the emergence of monocytes/macrophages from hESCs through steps that included the sequential formation of mixed progenitors, monocyte precursors, mature monocytes, and macrophages, as has been described for bone marrow monopoiesis.
To monitor this differentiation in detail, we combined CD14 and CD16 markers, which discriminate the 2 major types of monocytes, CD14+CD16− and CD14+CD16+ monocytes, in adult peripheral blood.29 These cells have been shown to exhibit distinct phenotypes and functions, although the CD14+CD16+ cell population seems quite heterogeneous. In addition, CD16 expression appears at the surface of CD14+CD16− adult peripheral blood monocytes when induced to differentiate into macrophages on M-CSF exposure (unpublished data). In hESC- and fetal liver–derived cultures, these 2 markers identified 2 populations that were either CD14lowCD16− or CD14highCD16+. The first population clearly consisted of precursors capable of proliferating and giving rise to the second population in the presence of M-CSF, GM-CSF, IL-3, or a cocktail of cytokines. The CD14highCD16+ cells formed a homogenous population of monocytes and macrophages that did not proliferate. Interestingly, whatever the cytokine used and whatever the time in culture, a CD14highCD16− cell population was not observed. In contrast, in adult blood, CD14highCD16− monocytes represent the major blood population and the CD14highCD16+ subset accounts for only 10% of all adult monocytes.18
The difference in phenotype between embryonic/fetal and adult blood monocytes could reflect developmental changes in the monocyte/macrophage lineage. To explore this possibility, we compared the differentiation pattern of CD34+ cells from human fetal liver and from adult blood into the monocyte/macrophage lineage using a similar cocktail of hematopoietic cytokines. This approach demonstrated that monocyte/macrophage differentiation from hESCs and fetal liver were extremely close and occurred from a CD14lowCD16− progenitor; this was in contrast to adult cultures, in which macrophage differentiation occurred through a CD14highCD16− differentiation step. In addition, we confirmed results obtained on mouse embryonic monopoiesis demonstrating the redundancy of M-CSF, GM-CSF, and IL-3.14,35 In mice, a bone marrow progenitor with a M-CSFR+CXCR3+Flt3+ phenotype, termed MDP, gives rise to several monocyte/macrophage subsets and dendritic cells.4,31 The hESC-derived CD14lowCD16− cells also expressed CD115 (M-CSFR), FLT3, and CXCR3 and gave rise to both dendritic cells and macrophages; therefore, these cells could be the equivalent of the MDP murine progenitor. Confirmation of this assumption will require further experiments performed at the unicellular level.
Our findings underscore the presence of a developmental regulation in human monocyte/macrophage differentiation. Mouse embryonic macrophages are specialized in phagocytosis, tissue remodeling, and angiogenesis.15,17 In the present study, we show that the CD14+CD16+ embryonic and fetal monocytes/macrophages demonstrated a CD62L−CCR2−CCR5+CX3CR1+ phenotype that overlapped with that of adult nonclassic monocytes.3 In addition, they exhibited a positive ratio between anti-inflammatory and proinflammatory cytokines, suggesting M2 polarization, and responded poorly to IFN-γ and LPS. Our finding that embryonic/fetal macrophages secrete higher amounts of TGF-β1 suggests the presence of additional paracrine/autocrine mechanisms that control the polarization of immune cells, contributing to the maternal-fetal tolerance during embryonic development.
M2 markers identified in embryonic and fetal monocytes/macrophages include IGF1, LYVE1, MRC1, STAB1, and MMP12, which are expressed on their murine counterparts and on monocytes expressing the angiopoietin receptor Tie2 (TEMs) and human tumor-associated macrophages.17 These latter cells promote tumor development by providing growth factors and remodeling factors that facilitate invasion and metastasis.14,36,37 The cytokines secreted at the highest level by the embryonic subset were either those regulating the degradation of extracellular matrix, such as MMPs, Serpins, and TIMPs, or pleiotropic cytokines such as IGFs, NRG1, TGF-β, which are known to act at the different stages of morphogenesis in the mouse embryo.14,38,39 The impressive production of a wide range of metalloproteases suggests that embryonic and fetal macrophages may have specific trophic functions to regulate tissue architecture and cell migration40,41 ; hESC- and fetal liver–derived macrophages particularly expressed a high level of MMP12, a differential marker between embryonic TEM and other embryonic macrophages.17 In addition, hESC-derived monocytes/macrophages synthesized a large pattern of factors involved in angiogenesis that could even be up-regulated in a hypoxic embryonic environment. One of the functions of adult TEMs is to promote angiogenesis in xenograft transplants.18 In a similar assay, we could not demonstrate a significant pro-angiogenic activity using our hESC- and fetal liver–derived “TEMs.” This negative result may have 3 explanations: (1) the assay used, because in our study a profuse angiogenesis was observed in the control group; (2) the exit of embryonic macrophages from the tumor; or (3) apoptosis of embryonic macrophages after cytokine starvation. Nevertheless, embryonic cells promoted the enlargement of the tumor vessel lumen, which might be related to MMP secretion.38,39 Like adult macrophages, embryonic monocytes exhibited phagocytosis functions required for development.42
It remains to be determined whether monocyte/macrophage subsets and evolution with time reflect the plasticity of a unique cell population or the presence of distinct precursors. According to one hypothesis, plasticity could be generated by epigenetic controls restricting monocyte/macrophage functions to tissue remodeling in the embryo and the fetus, as recapitulated by tumor-associated macrophages, and widening monocyte/macrophage functions to inflammation in adults. A second hypothesis states that distinct precursors would arise sequentially during development, with the first wave being more specialized in M2 functions. Monocyte/macrophage differentiation from hESCs might be a powerful tool with which to precisely understand the developmental mechanisms of monocyte/macrophage heterogeneity, and may also offer a valuable experimental model for in vitro studies of mechanisms sustaining tumor development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Naïma Beniklef for blood monocyte samples; Assistance Publique des Hôpitaux de Paris-Hôpital Pitié Salpetriere Cellular Therapy Laboratory, for CD34+ cell samples; Yann Lecluse and Philippe Rameau for cell sorting; Patrick Gonin for statistical analysis; Laetitia Chaussumier and Marcella Mori for help in some experiments; Karim Maloum and Orianne Wagner-Ballon for cell morphologic analysis; Olivia Bawa for tumor histologic analysis; Yann Monnet for technical assistance with animal models; Denis Arnaud for technical support in some experiments; and Nathalie Droin and Françoise Wendling for reading and improving the manuscript.
This work was supported by grants from the Médicen Paris région (consortium Ingecell, Paris France), Agence Nationale pour la Recherche Blanc (Megon), and Inserm. O.K. was supported by a fellowship from the Ministère de la Recherche and the French Society of Hematology and S.B. by the Institut national du cancer (INCa).
Authorship
Contribution: O.K. designed and performed experiments, analyzed data, and wrote the manuscript; A.D.S. and S.H. performed culture experiments; B.G. and P.O. performed xenograft model experiments; T.R. performed quantitative RT-PCR experiments; M.R. provided technical support; J.E.-B. performed phagocytosis experiments; A.-L.D. provided fetus samples; S.B. performed electron microscopy cell analysis; B.L. performed protein-analysis experiments; E.S. discussed results; W.V. supervised the research and wrote the manuscript; and F.N. conceived of and designed the experiments, supervised the research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Françoise Norol, APHP, Service de Biotherapie, Groupe Hôspitalier Pitié Salpétrière, 83 Boulevard de l'Hôpital, 75013 Paris, France; e-mail: f.norol@psl.aphp.fr.