Abstract
X-linked lymphoproliferative disease (XLP1) is a rare immunodeficiency characterized by severe immune dysregulation and caused by mutations in the SH2D1A/SAP gene. Clinical manifestations are varied and include hemophagocytic lymphohistiocytosis (HLH), lymphoma and dysgammaglobulinemia, often triggered by Epstein-Barr virus infection. Historical data published before improved treatment regimens shows very poor outcome. We describe a large cohort of 91 genetically defined XLP1 patients collected from centers worldwide and report characteristics and outcome data for 43 patients receiving hematopoietic stem cell transplant (HSCT) and 48 untransplanted patients. The advent of better treatment strategies for HLH and malignancy has greatly reduced mortality for these patients, but HLH still remains the most severe feature of XLP1. Survival after allogeneic HSCT is 81.4% with good immune reconstitution in the large majority of patients and little evidence of posttransplant lymphoproliferative disease. However, survival falls to 50% in patients with HLH as a feature of disease. Untransplanted patients have an overall survival of 62.5% with the majority on immunoglobulin replacement therapy, but the outcome for those untransplanted after HLH is extremely poor (18.8%). HSCT should be undertaken in all patients with HLH, because outcome without transplant is extremely poor. The outcome of HSCT for other manifestations of XLP1 is very good, and if HSCT is not undertaken immediately, patients must be monitored closely for evidence of disease progression.
Introduction
X-linked lymphoproliferative disease (XLP) is a rare primary immunodeficiency first described in 1975 by Purtilo1 and characterized by severe immune dysregulation often after viral infection (typically with Epstein-Barr virus [EBV]). Since XLP was first described, our understanding of the molecular and cellular pathogenesis of the disease has greatly improved. However, clinically, it is still difficult to determine optimal management and prognosis for patients due to the variability of clinical presentation, lack of genotype-phenotype correlation, and rarity of the disease. Purtilo established an XLP registry in 1980, and by 1995 more than 270 boys had been identified in 80 kindreds.2 To date this registry has provided the only data on clinical phenotype and prognosis for this patient group. Overall mortality in this group was 75%, with 70% of boys succumbing before 10 years of age. However, current outcomes for XLP may be very different due to the availability of unambiguous molecular diagnosis, improved viral monitoring, and the improvement in treatment regimens for disease manifestations.
XLP affects 1 to 3 million boys,3,4 and most commonly presents in childhood or early adolescence. Presentation may be acute in the case of fulminant infectious mononucleosis (FIM)/hemophagocytic lymphohistiocytosis (HLH) or lymphoma or less aggressive with dysgammaglobulinemia or recurrent infections. Patients often manifest more than one phenotype and may progress from one phenotype to another, for example presenting with hypogammaglobulinemia and progressing to lymphoma, and different clinical features are often present in families highlighting the lack of genotype-phenotype correlation. Other rare but well-described presenting features include aplastic anemia, vasculitis, and chronic gastritis.2,5-8 It is now known that the clinical syndrome of XLP arises from 2 different genetic defects in SH2D1A (XLP1, by far the most common and the focus of this report) and the BIRC/XIAP gene (XLP2). The gene responsible for XLP1 is the SH2D1A gene found on the X chromosome at position Xq25,9-11 which encodes the cytoplasmic protein SAP (signaling lymphocyte activation molecule or SLAM-associated protein). SAP is a key regulator of normal immune function in T cells,12-14 natural killer (NK) cells,15-18 NKT cells,19,20 and possibly B cells,21 and defects in this protein lead to the varied immune defects described in XLP1 patients.20,22 Humoral defects seen in this disease are thought to arise from impaired CD4+ T-cell interaction with B cells and not an intrinsic B-cell deficit.23
Although it has always been presumed that EBV infection plays a crucial role in the development of clinical features in XLP1 patients, it is now clear that a proportion of boys are EBV negative at presentation and remain so. Indeed, 10% of patients have immunological abnormalities before any evidence of EBV exposure.4,24 XLP1 is therefore a disorder of immune dysregulation rather than a disorder specifically associated with EBV infection.
Before 1994, acute management of FIM and HLH included antiviral medications, high-dose intravenous immunoglobulin (Ig), immunosuppressants, and other immune modulators such as interferon-α. These treatments proved disappointing25 and the XLP registry data showed a survival of only 4% for boys presenting with these manifestations. Improved chemotherapy regimes for lymphoma and immunosuppressive protocols to treat HLH (including rituximab) may reduce the mortality rate for XLP1 patients and allow stabilization before hematopoietic stem cell transplant (HSCT).26 Our report provides valuable outcome data collected since the introduction of current HLH treatment protocols, focusing on XLP1 patients with mutations in the SH2D1A gene.
Allogeneic HSCT remains the only curative option for XLP1 at present although large scale outcome studies are not available. Recently, Lankester et al reviewed 14 cases in the literature who had undergone HSCT and found an overall survival of 71% (10/14) with little evidence of EBV reactivation and posttransplant lymphoproliferative disease.27 We describe here outcome data for a much larger cohort of patients transplanted since 1997.
There is no consensus on whether clinically stable XLP1 patients should undergo HSCT as the natural history of the disease is so variable, even within the same family. Treatment and management of the disease is severely hampered by the lack of data of a large cohort of patients and previously published outcome data are based on historical data, which may represent patients with conditions other than XLP1 as inclusion was based on clinical and not genetic diagnosis. Also, little recent data exist for patients who remain untransplanted. Hence, we describe a large cohort of genetically defined XLP1 patients collected from centers worldwide. The data presented will allow for better counseling of affected families regarding prognosis and management options, particularly in relation to timing of transplant.
Methods
Data collection
Questionnaires regarding patient demographics, transplant characteristics, and outcome were sent to centers worldwide identified through the European Society for Immunodeficiencies/European Bone Marrow Transplantation Registry, published case reports or centers known to perform pediatric HSCT. Retrospective analysis was performed using data collected for 91 patients from 32 centers worldwide. The number of cases from each center varied between 1 and 27 but was on average 1-2 cases. Patients included in this study were born between 1941 and 2005; 63 were born in or after 1990 (24 untransplanted patients and 39 transplanted patients). Only patients with a confirmed mutation in the SH2D1A gene were included in this series. Patients with mutations in other XLP-associated genes such as XIAP/BIRC-4 were excluded, as were patients with abnormal SAP expression but no confirmed mutation in SH2D1A. EBV status was determined by polymerase chain reaction to avoid variable serology results in XLP1 patients and especially in those with dysgammaglobulinemia. Questionnaires offered reporting of FIM and HLH separately; thus, some centers with experience in this area reported patient data accordingly, and it is presented as such.
Management of HLH and lymphoma
Patients who presented with HLH were managed predominantly in accordance with HLH 94 or HLH 2004 protocols. Additional or alternative treatment included antiviral therapy (aciclovir, ganciclovir, or foscarnet, n = 6), high-dose intravenous immunoglobulin (n = 9), immunosuppression (steroids, cyclosporine, and etoposide, n = 12), or anti-CD20 antibody (rituximab, n = 10). Intrathecal therapy was used where central nervous system involvement was suspected. Ten patients who proceeded to transplant received rituximab therapy before transplant, either as treatment for HLH or during conditioning.
Regimes for the treatment of lymphoma varied in line with appropriate national guidelines (eg, COPAD [cyclophosphamide, vincristine, prednisone, and doxorubicin]) study, Berlin-Frankfurt-Munster Group, Associazione Italiana Ematologia Oncologia Pediatrica, or United Kingdom Children's Cancer Study Group guidelines) and only occasionally involved surgical management.
Statistical analysis
Kaplan-Meier curves were used to analyze survival figures. The log rank test (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests were used to compare survival between different groups. Statistical analysis including hazard ratio calculation was performed using GraphPad Prism Version 5.00 for Windows.
Results
Data from 91 patients (64 pedigrees) in 32 centers worldwide were included in this report. The overall survival of XLP1 patients was 71.4% (65/91), and patients displayed a heterogeneous clinical phenotype. Due to the heterogeneity of the group, data were analyzed according to presentation with HLH, EBV status, and whether patients had received HSCT, allowing characterization of outcome after transplant.
Spectrum of XLP1 mutations
In keeping with previous publications, no genotype/phenotype correlation was evident, and the most frequently reported mutation involved the arginine residue at position 55 (exon 2) found in 11 patients from 9 different families. Detailed genetic information was available for 62 patients (50 pedigrees; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Exon 2 had the most mutations with missense mutations accounting for the majority but nonsense, frameshift, and splice site mutations were also reported. Large gene deletions (up to 11 Mb) including those involving the whole gene were identified in 5 families. Three of these larger deletions were associated with gastrointestinal symptoms of colitis and gastritis. Such symptoms were not found in patients with other mutations apart from a patient with diarrhea as a feature (missense mutation exon 1, 62 T > C). In a further 29 patients, detailed genetic data were not supplied but a SAP/SH2D1A gene defect was confirmed by the documenting center.
Clinical manifestations of XLP1
Table 1 shows the presenting features of disease as well as features of disease manifesting throughout the course of the condition. HLH remained the most common presenting feature (39.6%), although dysgammaglobulinemia was the manifestation seen most commonly in patients during the course of the illness.
Presenting symptoms and features of XLP1 patients with associated mortality
| . | Incidence . | Mortality . |
|---|---|---|
| Presenting symptom | ||
| HLH | 31.9% | 65.5% |
| FIM | 7.7% | 14.3% |
| Lymphoma | 14.3% | 7.7% |
| Dysgammaglobulinemia | 22% | 5% |
| Family history of XLP1 alone | 16.5% | 20% |
| Other | 7.7% | 14.3% |
| Features occurring at any time | ||
| HLH | 35.2% | 65.6% |
| FIM | 9.9% | 22.2% |
| Lymphoma | 24.2% | 9% |
| Dysgammaglobulinemia | 50.5% | 13% |
| Other | 15.4% | 28.6% |
| . | Incidence . | Mortality . |
|---|---|---|
| Presenting symptom | ||
| HLH | 31.9% | 65.5% |
| FIM | 7.7% | 14.3% |
| Lymphoma | 14.3% | 7.7% |
| Dysgammaglobulinemia | 22% | 5% |
| Family history of XLP1 alone | 16.5% | 20% |
| Other | 7.7% | 14.3% |
| Features occurring at any time | ||
| HLH | 35.2% | 65.6% |
| FIM | 9.9% | 22.2% |
| Lymphoma | 24.2% | 9% |
| Dysgammaglobulinemia | 50.5% | 13% |
| Other | 15.4% | 28.6% |
Although clinical features have remained similar to previously published data,2 the survival associated with XLP1 is 71.4%, which is significantly improved over historical survival of 25%. The survival associated with different phenotypes has also changed significantly with mortality associated with HLH decreased from 96% to 65%, lymphoproliferative disease from 35% to 8%, and dysgammaglobulinemia from 55% to 5%.
Twenty-two patients suffered from malignant lymphoproliferative disease, with eighteen patients (81.8%) diagnosed with B-cell non-Hodgkin lymphoma mainly of the abdomen and cervical region. In 5 patients the disease was recurrent, with 1 patient experiencing a cerebral tumor. Only 1 patient was reported with cerebral T-cell lymphoma. Data on tumor histology is lacking in 3 patients.
Immunological abnormalities at diagnosis
Details of immune function were available for 57 patients, although in some cases, data were only available after the onset of disease manifestations that may have influenced immunoglobulin and lymphocyte subset levels. Immunoglobulin levels were recorded in 49 patients, and 32 of these showed varying degrees of abnormal immunoglobulin levels. Twelve children presented with neutropenia. Lymphocyte subset data were available for 47 patients; 19 showed a reduced percentage of B cells, 26 showed low NK cell numbers, and 12 had a reversed CD4:CD8 ratio.
Presentation with HLH
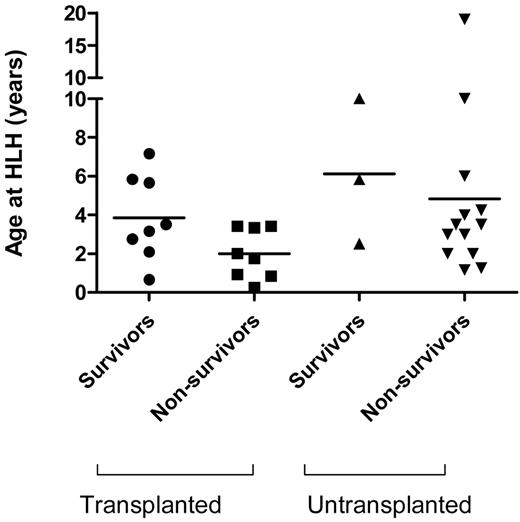
The mortality for patients presenting with HLH was 65.6%, with a median age at presentation of 3 years 2 months (range 8 months to 9 years). Of the 32 patients with HLH, 16 underwent transplant, of whom 8 survived (50%; Figure 1). Of those who did not receive a transplant, only 3 survived (18.8%), confirming previous reports that the prognosis for patients with HLH associated with a genetic defect is extremely poor and that HSCT is necessary.
Outcome of patients with HLH during course of disease. Survival of patients who present with HLH—patients who remain untransplanted have a poor survival outcome with only 18.8% survival. By contrast the survival of those who undergo transplant is higher at 50%.
Outcome of patients with HLH during course of disease. Survival of patients who present with HLH—patients who remain untransplanted have a poor survival outcome with only 18.8% survival. By contrast the survival of those who undergo transplant is higher at 50%.
EBV status
EBV status was documented in 79 patients showing that 51 (64.6%) were EBV positive at presentation or diagnosis (Table 2 and supplemental Figure 1). The median age of presentation in this group was 4 years (range 8 months to 40 years), and the overall mortality was 35.2% (18/51). There was no significant difference in mortality between patients with (35.2%) and without (28.6%) documented EBV infection. HLH/FIM was the most common feature in this group being seen in 35 patients (68.6%), with lymphoma present in 10 patients (19.6%), and dysgammaglobulinemia in 19 (37.2%). Nine EBV-positive patients had a family history of XLP1, and two others had a family history suggestive of an X-linked immunodeficiency. Of the 18 EBV-positive patients who died, the majority (14/18) died within 2 months of presentation due to disease progression. Three died in the early posttransplant period of infective complications and disease progression, and 1 died during treatment for lymphoma.
Characteristics of EBV-positive and EBV-negative XLP1 patients
| . | EBV positive (64.6%, n = 51) . | EBV negative (35.4%, n = 28) . |
|---|---|---|
| Median age at presentation | 4 y (8 mo-40 y) | 3 y (0-31 y) |
| Family history of XLP1 | 17.6% | 42.9% |
| HLH | 51% | 21.4% |
| FIM | 17.6% | |
| Lymphoma | 19.6% | 25% |
| Dysgammaglobulinemia | 37.2% | 51.8% |
| Mortality | 35.2% | 28.6% |
| Median age at death | 3 y 6 mo (14 mo-21 y) | 5 y 11 mo (20 mo-31 y) |
| . | EBV positive (64.6%, n = 51) . | EBV negative (35.4%, n = 28) . |
|---|---|---|
| Median age at presentation | 4 y (8 mo-40 y) | 3 y (0-31 y) |
| Family history of XLP1 | 17.6% | 42.9% |
| HLH | 51% | 21.4% |
| FIM | 17.6% | |
| Lymphoma | 19.6% | 25% |
| Dysgammaglobulinemia | 37.2% | 51.8% |
| Mortality | 35.2% | 28.6% |
| Median age at death | 3 y 6 mo (14 mo-21 y) | 5 y 11 mo (20 mo-31 y) |
Twenty-eight patients were EBV negative at presentation or diagnosis. The median age of presentation for this group was 3 years (range birth to 31 years). Family history of XLP1 was the presenting feature for 12 patients, and a further 7 patients described a family history suggestive of an X-linked immunodeficiency or lymphoma. There was a higher rate of dysgammaglobulinemia (51.8%) in this group. Lymphoma was present in 7 patients. Fewer EBV negative patients presented with HLH/FIM, and this may suggest that at least for this manifestation a viral trigger is important. Information was sought on other viral infectious agents including cytomegalovirus and adenovirus, but data were not available for most patients. Other clinical features included aplastic anemia in 3 patients and vasculitis in 2 patients. The mortality for this EBV negative group was 28.6% (8/28); 3 patients died shortly after presentation before HSCT with central nervous system vasculitis (2) and HLH with enterococcal sepsis (1). One patient died 11 years after presentation following a complex course, and a further 4 patients died in the early posttransplant period (described in Table 5).
HSCT for XLP1
HSCT was undertaken in 22 centers (range of patients/center: 1-7) between 1997 and 2009 (Table 3). Forty-six transplants were performed on 43 patients, and the median age at transplant was 6.25 years (range 8 months to 19 years); 1 patient who had undergone a haploidentical transplant received a CD34+ selected boost 1 year after initial transplant. One patient received an allogeneic HSCT to treat lymphoma before a diagnosis of XLP1 was established. Most patients received bone marrow or peripheral blood stem cells, and only 2 patients received umbilical cord HSCT. Donor grafts were from human leukocyte antigen-matched family donors in 14 cases, mismatched family donors or matched unrelated grafts in 28 cases, and haploidentical donors in 4 cases. Half of the transplant procedures (23/46) were performed using myeloablative conditioning regimes including combinations of busulfan 12-20 mg/kg, cyclophosphamide 50-200 mg/kg, and total body irradiation 5-12 Gy. The other half of procedures used nonmyeloablative conditioning regimes consisting of fludarabine (30 mg/kg), melphalan (70-140 mg/kg), busulphan (4-12 mg/kg), or total body irradiation (3-5 Gy). Twenty-six patients received additional serotherapy with alemtuzumab, anti-thymocyte globulin, anti-CD3 antibody, and anti-CD20 antibody (rituximab). Graft-versus-host disease (GVHD) prophylaxis regimes differed between centers, but mostly involved combinations of cyclosporin with methotrexate, mycophenolate mofetil, steroids, and tacrolimus. T-cell depletion of the graft was used in 1 case.
Characteristics of XLP1 patients receiving allogeneic HSCT
| . | Percentage . | Number . | 1-y survival . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|---|
| XLP1 features | ||||||
| Previous HLH | 37.2% | 16/43 | 50% | 23.93 | 5.31-108.0 | < .0001 |
| Previous NHL | 27.9% | 12/43 | 74.2% | 0.23 | 0.05-1.06 | .06 |
| Previous dysgammaglobulinemia | 46.5% | 20/43 | 80% | 1.2 | 0.29-4.96 | .77 |
| EBV+ | 51.2% | 21/41 | 75% | 1.37 | 0.36-5.3 | .65 |
| Age at HSCT | Mean 7 y (8 mo to 19 y 7 mo) | |||||
| 0-2 y | 9.3% | 4/43 | 75% | 5.75 | 0.11-302.1 | .38 |
| 2-5 y | 34.9% | 15/43 | 78.6% | 3.61 | 0.18-71.76 | .40 |
| 5-15 y | 48.8% | 21/43 | 85.7% | 3.16 | 0.11-90.83 | .50 |
| > 15 y | 7% | 3/43 | 100% | |||
| Year of HSCT | ||||||
| < 2000 | 7.0% | 3/43 | 66.7% | |||
| 2000-2005 | 37.2% | 16/43 | 87.5% | |||
| 2005-2009 | 55.8% | 24/43 | 79.2% | |||
| Donor Type | ||||||
| MSD, MFD | 30.4% | 14/46 | 91.77% | |||
| MUD, mMFD, mMUD | 60.9% | 28/46 | 77.8% | 0.42 | 0.08-2.07 | .27 |
| Haplo | 8.7% | 4/46 | 75% | 0.24 | 0.01-6.58 | .4 |
| Source | ||||||
| Bone marrow | 58.5% | 24/41* | 82.6% | |||
| Peripheral blood | 36.6% | 15/41* | 92.9% | |||
| Umbilical cord | 4.9% | 2/41* | 50% | |||
| Conditioning | ||||||
| MA | 50% | 23/46 | 82.9% | |||
| NMA | 50% | 23/46 | 78.9% | 1.25 | 0.30-5.2 | .77 |
| Serotherapy | 30.4% | 14/46 | ||||
| GVHD | 50% | 19/38 | ||||
| Grade 1 | 18.4% | 7/38 | ||||
| Grade 2-3 | 26.3% | 10/38 | ||||
| Grade 4 | 5.3% | 2/38 | ||||
| Chronic | 5.3% | 2/38 | ||||
| Chimerism | ||||||
| Full (> 98%) | 92% | 35/38 | 100% | |||
| Mixed | 8% | 3/38 | 88.8% | 2.98 | 0.06-151.0 | .59 |
| Replacement IVIg | 20% | 7/35† | ||||
| Alive | 81.4% | 35/43 | ||||
| Follow up | 6 wk to 148 mo |
| . | Percentage . | Number . | 1-y survival . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|---|
| XLP1 features | ||||||
| Previous HLH | 37.2% | 16/43 | 50% | 23.93 | 5.31-108.0 | < .0001 |
| Previous NHL | 27.9% | 12/43 | 74.2% | 0.23 | 0.05-1.06 | .06 |
| Previous dysgammaglobulinemia | 46.5% | 20/43 | 80% | 1.2 | 0.29-4.96 | .77 |
| EBV+ | 51.2% | 21/41 | 75% | 1.37 | 0.36-5.3 | .65 |
| Age at HSCT | Mean 7 y (8 mo to 19 y 7 mo) | |||||
| 0-2 y | 9.3% | 4/43 | 75% | 5.75 | 0.11-302.1 | .38 |
| 2-5 y | 34.9% | 15/43 | 78.6% | 3.61 | 0.18-71.76 | .40 |
| 5-15 y | 48.8% | 21/43 | 85.7% | 3.16 | 0.11-90.83 | .50 |
| > 15 y | 7% | 3/43 | 100% | |||
| Year of HSCT | ||||||
| < 2000 | 7.0% | 3/43 | 66.7% | |||
| 2000-2005 | 37.2% | 16/43 | 87.5% | |||
| 2005-2009 | 55.8% | 24/43 | 79.2% | |||
| Donor Type | ||||||
| MSD, MFD | 30.4% | 14/46 | 91.77% | |||
| MUD, mMFD, mMUD | 60.9% | 28/46 | 77.8% | 0.42 | 0.08-2.07 | .27 |
| Haplo | 8.7% | 4/46 | 75% | 0.24 | 0.01-6.58 | .4 |
| Source | ||||||
| Bone marrow | 58.5% | 24/41* | 82.6% | |||
| Peripheral blood | 36.6% | 15/41* | 92.9% | |||
| Umbilical cord | 4.9% | 2/41* | 50% | |||
| Conditioning | ||||||
| MA | 50% | 23/46 | 82.9% | |||
| NMA | 50% | 23/46 | 78.9% | 1.25 | 0.30-5.2 | .77 |
| Serotherapy | 30.4% | 14/46 | ||||
| GVHD | 50% | 19/38 | ||||
| Grade 1 | 18.4% | 7/38 | ||||
| Grade 2-3 | 26.3% | 10/38 | ||||
| Grade 4 | 5.3% | 2/38 | ||||
| Chronic | 5.3% | 2/38 | ||||
| Chimerism | ||||||
| Full (> 98%) | 92% | 35/38 | 100% | |||
| Mixed | 8% | 3/38 | 88.8% | 2.98 | 0.06-151.0 | .59 |
| Replacement IVIg | 20% | 7/35† | ||||
| Alive | 81.4% | 35/43 | ||||
| Follow up | 6 wk to 148 mo |
Data missing on 5 transplants, 1 died during conditioning.
Three patients < 1 year after transplant.
CI indicates confidence interval; HR, hazard ratio; MSD, matched sibling donor; MFD, matched family donor; MUD, matched unrelated donor; mMFD, mismatched family donor; mMUD, mismatched unrelated donor; Haplo, haploidentical transplant; MA, myeloablative; and NMA, nonmyeloablative.
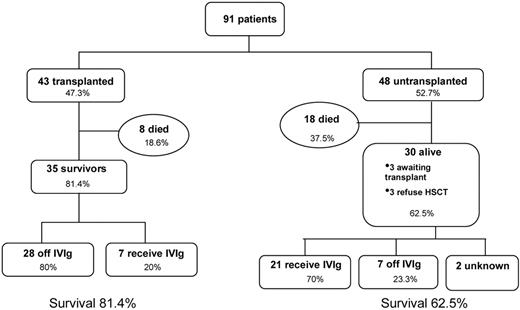
Outcome for XLP1 patients who received allogeneic HSCT was good with 81.4% surviving the procedure (35/43) with a median follow up of 52 months. The majority of these patients (28/35 survivors) required no ongoing immunoglobulin replacement therapy. Tables 3 and 4 highlight details of transplanted patients, and Figure 2 describes survival according to several factors.
Details of XLP1 patients surviving allogeneic HSCT
| Year of HSCT . | EBV . | HLH . | Age at HSCT . | Donor . | Conditioning/serotherapy/graft manipulation . | GVHD prophylaxis . | GVHD . | Chimerism . | Follow up (mo) . | Ig . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | NK | 7 y | MSD | Cy, TBI | MTX, CSA | 1 S* | 100% | 148 | ||
| 1998 | 1 y | MUD | Bu, Cy, ATG | MTX, CSA, P | 1 S | 100% | 133 | |||
| 2000 | + | 4 y | MUD | Bu, Cy, Campath | CSA | 2 S | 100% | 102 | ||
| 2000 | + | Yes | 3 y | mMUD | Bu, Cy | MTX, CSA | 2 S, L | 100% | 107 | |
| 2001 | 4 y | MUD | Flu, Melph, ATG, TBI | MMF, CSA | 100% | 102 | ||||
| 2001 | + | 10 y | MSD | Bu, Cy, VP-16 (NHL) | MTX, CSA | 2-3 GI | 100% | 99 | ||
| 2001 | 4 y | MSD | Bu, Cy | CSA | 2 S | 100% | 95 | |||
| 2002 | 13 y | MSD | Thio, Flu, ATG | CSA | 100% | 88 | ||||
| 2002 | + | 7 y | MUD | Bu, Cy, ATG | MTX, CSA | 1 S | 100% | 85 | ||
| 2002 | 3 y | MUD | Bu, Cy, ATG | MTX, CSA | 100% | 84 | ||||
| 2003 | 8 mo | mMUD | Flu, Melph, ATG, TBI | TAC, MTX, P | S | 100% | 79 | |||
| 2003 | 19 y | MSD | Thio, Flu, ATG | CSA | 100% | 71 | ||||
| 2003 | 11 y | mMUD | Flu, Melph, ATG | MMF, CSA | 100% | 68 | ||||
| 2004 | + | 5 y | MSD | Bu, Cy | MTX, CSA | 20% PBMC | 66 | |||
| 2004 | + | 12 y | mMFD | Flu, Melph, Campath, 34+ | MMF, CSA | 4 S, L* | 100% | 62 | Y | |
| 2004 | + | 8 y | mMUD | Flu, Melph, Campath | CSA | 2-3 S, GI | 100% | 57 | ||
| 2005 | + | Yes | 2 y | Haplo | Bu, Cy, ATG, 34+ | CSA | 100% | 54 | Y | |
| 2005 | 2 y | Haplo | Bu, Cy, ATG, 34+, top up 1 year | CSA | 88% PBMC 97% M | 50 | Y | |||
| 2005 | 12 y | mMUD | Flu, Melph, Campath, 34+ | MMF, CSA | 3 S | 100% | 46 | |||
| 2005 | 18 y | MUD | NK | NK | 46 | NK | ||||
| 2006 | 5 y | MSD | Bu, Cy | CSA | 1 G, 3 S | 100% | 42 | |||
| 2006 | NK | 2 y | MUD | Bu, Flu, Campath | MTX | 100% | 42 | |||
| 2006 | + | 7 y | Haplo | Flu, Melph, Thio, OKT3, ATG | 100%, 75% CD3 | 39 | ||||
| 2006 | + | Yes | 1 y | MUD | Flu, Melph, Ritux | CSA | 1 S | 5% | 38 | |
| 2006 | + | 11 y | MSD | Bu, Cy, ATG | MTX, CSA | 100% | 35 | |||
| 2006 | 4 y | MUD | Bu, Cy, Campath | MMF, CSA | 1 S | 100% | 33 | |||
| 2007 | + | Yes | 6 y | MSD | Bu, Cy | MTX, CSA | 99% | 30 | ||
| 2007 | + | Yes | 7 y | MSD | Bu, Cy | CSA | 3 S, L, GI | 100% | 29 | |
| 2007 | NK | 7 y | MUD | Flu, Melph, TBI | TAC, MTX | 98% | 27 | |||
| 2007 | 7 y | MSD/mMUD | Bu, Cy | CSA | 2 S, GI | 100% | 26 | |||
| 2008 | 17 y | MFD | Flu, Melp, Campath | MMF, CSA | 100% | 13 | ||||
| 2008 | Yes | 3 y | MUD | Bu, Flu | TAC, MTX | 100% | 9 | Y | ||
| 2009 | + | 7 y | MUD | Bu, Cy | 100% | 6 | Y | |||
| 2009 | + | Yes | 6 y | mMUD | Flu, Melph, Campath | CSA, MMF | 1 S | 100% | 5 | Y |
| 2009 | + | Yes | 3 y | MUD | Thio, Cy, ATG | CSA, P | 100% | 4 | Y |
| Year of HSCT . | EBV . | HLH . | Age at HSCT . | Donor . | Conditioning/serotherapy/graft manipulation . | GVHD prophylaxis . | GVHD . | Chimerism . | Follow up (mo) . | Ig . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | NK | 7 y | MSD | Cy, TBI | MTX, CSA | 1 S* | 100% | 148 | ||
| 1998 | 1 y | MUD | Bu, Cy, ATG | MTX, CSA, P | 1 S | 100% | 133 | |||
| 2000 | + | 4 y | MUD | Bu, Cy, Campath | CSA | 2 S | 100% | 102 | ||
| 2000 | + | Yes | 3 y | mMUD | Bu, Cy | MTX, CSA | 2 S, L | 100% | 107 | |
| 2001 | 4 y | MUD | Flu, Melph, ATG, TBI | MMF, CSA | 100% | 102 | ||||
| 2001 | + | 10 y | MSD | Bu, Cy, VP-16 (NHL) | MTX, CSA | 2-3 GI | 100% | 99 | ||
| 2001 | 4 y | MSD | Bu, Cy | CSA | 2 S | 100% | 95 | |||
| 2002 | 13 y | MSD | Thio, Flu, ATG | CSA | 100% | 88 | ||||
| 2002 | + | 7 y | MUD | Bu, Cy, ATG | MTX, CSA | 1 S | 100% | 85 | ||
| 2002 | 3 y | MUD | Bu, Cy, ATG | MTX, CSA | 100% | 84 | ||||
| 2003 | 8 mo | mMUD | Flu, Melph, ATG, TBI | TAC, MTX, P | S | 100% | 79 | |||
| 2003 | 19 y | MSD | Thio, Flu, ATG | CSA | 100% | 71 | ||||
| 2003 | 11 y | mMUD | Flu, Melph, ATG | MMF, CSA | 100% | 68 | ||||
| 2004 | + | 5 y | MSD | Bu, Cy | MTX, CSA | 20% PBMC | 66 | |||
| 2004 | + | 12 y | mMFD | Flu, Melph, Campath, 34+ | MMF, CSA | 4 S, L* | 100% | 62 | Y | |
| 2004 | + | 8 y | mMUD | Flu, Melph, Campath | CSA | 2-3 S, GI | 100% | 57 | ||
| 2005 | + | Yes | 2 y | Haplo | Bu, Cy, ATG, 34+ | CSA | 100% | 54 | Y | |
| 2005 | 2 y | Haplo | Bu, Cy, ATG, 34+, top up 1 year | CSA | 88% PBMC 97% M | 50 | Y | |||
| 2005 | 12 y | mMUD | Flu, Melph, Campath, 34+ | MMF, CSA | 3 S | 100% | 46 | |||
| 2005 | 18 y | MUD | NK | NK | 46 | NK | ||||
| 2006 | 5 y | MSD | Bu, Cy | CSA | 1 G, 3 S | 100% | 42 | |||
| 2006 | NK | 2 y | MUD | Bu, Flu, Campath | MTX | 100% | 42 | |||
| 2006 | + | 7 y | Haplo | Flu, Melph, Thio, OKT3, ATG | 100%, 75% CD3 | 39 | ||||
| 2006 | + | Yes | 1 y | MUD | Flu, Melph, Ritux | CSA | 1 S | 5% | 38 | |
| 2006 | + | 11 y | MSD | Bu, Cy, ATG | MTX, CSA | 100% | 35 | |||
| 2006 | 4 y | MUD | Bu, Cy, Campath | MMF, CSA | 1 S | 100% | 33 | |||
| 2007 | + | Yes | 6 y | MSD | Bu, Cy | MTX, CSA | 99% | 30 | ||
| 2007 | + | Yes | 7 y | MSD | Bu, Cy | CSA | 3 S, L, GI | 100% | 29 | |
| 2007 | NK | 7 y | MUD | Flu, Melph, TBI | TAC, MTX | 98% | 27 | |||
| 2007 | 7 y | MSD/mMUD | Bu, Cy | CSA | 2 S, GI | 100% | 26 | |||
| 2008 | 17 y | MFD | Flu, Melp, Campath | MMF, CSA | 100% | 13 | ||||
| 2008 | Yes | 3 y | MUD | Bu, Flu | TAC, MTX | 100% | 9 | Y | ||
| 2009 | + | 7 y | MUD | Bu, Cy | 100% | 6 | Y | |||
| 2009 | + | Yes | 6 y | mMUD | Flu, Melph, Campath | CSA, MMF | 1 S | 100% | 5 | Y |
| 2009 | + | Yes | 3 y | MUD | Thio, Cy, ATG | CSA, P | 100% | 4 | Y |
Chronic GVHD.
PBMC indicates peripheral blood mononuclear cell; Flu, fludarabine; Melph, melphalan; 34+, CD34+ stem cell infusion; Bu, busulfan; Cy, cyclophosphamide; Thio, thiotepa; TBI, total body irradiation; CSA, cyclosporin A; MMF, mycophenalate mofetil; MTX, methotrexate; P, prednisolone; TAC, tacrolimus; S, skin; GI, gastrointestinal; L, lung; and Ig, replacement immunoglobulin.
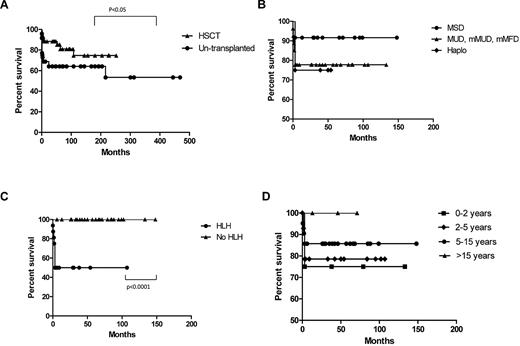
Survival in XLP1 related to different variables. (A) Overall survival of transplanted versus untransplanted patients. In the transplanted group this represents time from presentation and not transplant. (B) Survival according to donor source. (C) Survival after HSCT with relation to presence of HLH before transplant. (D) Survival according to age at transplant.
Survival in XLP1 related to different variables. (A) Overall survival of transplanted versus untransplanted patients. In the transplanted group this represents time from presentation and not transplant. (B) Survival according to donor source. (C) Survival after HSCT with relation to presence of HLH before transplant. (D) Survival according to age at transplant.
Sixteen patients were diagnosed with HLH before transplant and 12 patients had some form of lymphoproliferative disease (lymphoma). Only 51.2% of the cohort had documented evidence of EBV infection (by polymerase chain reaction) with survival rates in EBV+ patients similar to those without EBV infection (75% vs 80%). Most patients experienced some delay from first symptoms to diagnosis (average delay 2 years 7 months) but once a diagnosis of XLP1 was established time to transplant was generally less than 1 year. Median age at transplant was 6.25 years with a range of 8 months to 19 years.
Univariate analysis was performed to identify the major risk factors for survival after HSCT. The most important risk factor was prior HLH, which significantly decreased the survival outcome to 50%. A previous diagnosis of lymphoma had a near significant effect, but other variables were not shown to have a significant effect including importantly, previous evidence of EBV infection, the age at transplant, donor type, or the conditioning regime. It is also important to note that only patients who had HLH at some point before or during transplant died. Conversely, all patients without HLH (n = 27) survived the transplant procedure.
Half of the patients underwent a nonmyeloablative conditioning regime before HSCT and this did not impact on survival (nonmyeloablative vs myeloablative, 78.9% vs 82.9%) or long-term chimerism. More than 90% of patients achieved full donor chimerism, and those with a mixed or falling chimerism remained well with 1 patient still receiving replacement immunoglobulin.
Data were also collected on common posttransplant complications such as GVHD, infectious complications and toxicity attributable to chemotherapy. Half of the patients (50%) suffered from some form of GVHD; the majority of cases were grade 1-3 affecting the skin, liver, and gut. Two patients suffered grade 4 disease (of skin and liver), and 1 of these children died. Only 2 patients went on to develop chronic GVHD (see Table 3). One patient experienced both veno-occlusive disease and renal toxicity due to conditioning (busulfan, cyclophosphamide, and antithymocyte globulin), and this patient succumbed shortly after a haploidentical transplant.
In 3 patients with mixed chimerism in peripheral blood mononuclear cells, this remained stable in all but 1 patient, in whom it fell from 92% to 5%. However, this patient remains well 3 years posttransplant and does not require replacement immunoglobulin therapy. From this series, there is little evidence of viral reactivation posttransplant. Thirty-five patients are alive with 5 suffering some long-term effects including EBV viremia (managed with rituximab), bronchiectasis, autoimmune disease, chronic psoriasis, and neutropenia.
Eight patients did not survive after HSCT (see Table 5). Seven patients who died presented with HLH before HSCT (4/7 EBV+) compared with 8 of 35 survivors, but HLH was a feature of disease in all 8 nonsurvivors. The majority of nonsurvivors were ≤ 3 years old (5/8), and conditioning regime did not appear to play a role as 5/8 patients received a full myeloablative regime. The main cause of death in this group was sepsis, but disease progression accounted for 2 deaths. The 2 children dying with disease progression went into transplant with active disease; 1 died during conditioning and the other 3 days after HSCT. One further patient died 3 weeks after HSCT (7 months after presentation) from veno-occlusive disease (VOD), multiorgan failure, and renal toxicity attributable to chemotherapy. The remaining 5 patients died of sepsis (2 pseudomonal sepsis, 1 parainfluenza III infection, 1 with disseminated adenoviral infection, and 1 with EBV and fungal infection) within 3 months of HSCT.
Details of XLP1 patients not surviving allogeneic HSCT
| EBV . | HLH . | Age at HSCT, y . | Year of HSCT . | Donor . | Conditioning/serotherapy/graft manipulation . | GVHD prophylaxis . | GVHD . | Chimerism . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| + | Yes | 2 | 2005 | MMFD | Flu, TBI | N/A | Died during conditioning 6 wk from presentation | ||
| + | Yes | 3 | 2003 | MUD | Bu, Flu, Campath, Rituximab | CSA | Died 3 d after HSCT disease progression | ||
| Yes | 6 | 2005 | MMFD | Bu, TBI | MMF. MTX, P | Died 14 d after HSCT MDR pseudomonal sepsis | |||
| + | Yes | 3 | 2009 | Haplo | Bu, Cy, ATG | TCD | Died 3 wk after HSCT VOD, MOF, renal toxicity | ||
| + (after HSCT) | Yes | 5 | 2008 | mMUD (cord plus PBSC 4 months later) | Bu, Flu, ATG then Flu, TBI | TAC, P | 100% | Died 2 mo after second HSCT EBV, fungal, and ?PCP sepsis | |
| Yes | 3 | 1998 | MSD × 2 | Flu, Melph | CSA, P | 100% | Died 3 mo after HSCT Pseudomonas sepsis | ||
| + | Yes | 12 | 2003 | MUD | Bu, Cy, Flu, Campath | 4 S | 100% | Died 3 mo after HSCT disseminated adenovirus | |
| Yes | 1 | 2007 | MUD | Flu, Melph, ATG, 34+ | CSA | 2-3 S, L | 100% | Died 3 mo after HSCT paraflu III sepsis |
| EBV . | HLH . | Age at HSCT, y . | Year of HSCT . | Donor . | Conditioning/serotherapy/graft manipulation . | GVHD prophylaxis . | GVHD . | Chimerism . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| + | Yes | 2 | 2005 | MMFD | Flu, TBI | N/A | Died during conditioning 6 wk from presentation | ||
| + | Yes | 3 | 2003 | MUD | Bu, Flu, Campath, Rituximab | CSA | Died 3 d after HSCT disease progression | ||
| Yes | 6 | 2005 | MMFD | Bu, TBI | MMF. MTX, P | Died 14 d after HSCT MDR pseudomonal sepsis | |||
| + | Yes | 3 | 2009 | Haplo | Bu, Cy, ATG | TCD | Died 3 wk after HSCT VOD, MOF, renal toxicity | ||
| + (after HSCT) | Yes | 5 | 2008 | mMUD (cord plus PBSC 4 months later) | Bu, Flu, ATG then Flu, TBI | TAC, P | 100% | Died 2 mo after second HSCT EBV, fungal, and ?PCP sepsis | |
| Yes | 3 | 1998 | MSD × 2 | Flu, Melph | CSA, P | 100% | Died 3 mo after HSCT Pseudomonas sepsis | ||
| + | Yes | 12 | 2003 | MUD | Bu, Cy, Flu, Campath | 4 S | 100% | Died 3 mo after HSCT disseminated adenovirus | |
| Yes | 1 | 2007 | MUD | Flu, Melph, ATG, 34+ | CSA | 2-3 S, L | 100% | Died 3 mo after HSCT paraflu III sepsis |
PBSC indicates peripheral blood stem cell; Flu, fludarabine; Melph, melphalan; 34+, CD34+ stem cell infusion; Bu, busulphan; Cy, cyclophosphamide; Thio, thiotepa; TBI, total body irradiation; CSA, cyclosporine A; MMF, mycophenalate mofetil; MTX, methotrexate; P, prednisolone; TAC, tacrolimus; TCD, T-cell depletion; S, skin; L, lung; VOD, veno-occlusive disease; MOF, mullti-organ failure; MDR, multidrug resistant; and PCP, Pneumocystis jiroveci.
Untransplanted patients
Data were available for 48 patients who did not receive HSCT (Table 6); 30 are alive, 4 of whom are actively awaiting transplant, and 3 who refused HSCT. One patient had received an autologous HSCT before diagnosis with XLP1, and this patient's data were analyzed as though untransplanted. Less detailed information was available for this set of patients compared with those receiving HSCT. This may be because some patients died before EBV status and immune function could be established and any first symptoms may not have been recognized as a manifestation of XLP1. From data available, median age at presentation was 5 years, and delay in diagnosis ranged from a few weeks to 32 years.
Characteristics of XLP1 patients not receiving HSCT
| . | . | Number . |
|---|---|---|
| Age at first symptom | 8 y 8 mo (6 mo-40 y) | |
| Age at death | 7.5 y (1-31 y) | |
| Time from presentation to death | 17.3 mo (1 NK) 9 d-18 y | |
| Time from first symptom (in those patients alive) | 12 y (1 NK) 1-39 y | |
| Presenting symptom | ||
| HLH | 31.3% | 15/48 |
| FIM | 10.4% | 5/48 |
| Lymphoma | 16.7% | 8/48 |
| Dysgammaglobulinemia | 29.2% | 14/48 |
| Other | 12.5% | 6/48 |
| Features | ||
| HLH | 33.3% | 16/48 |
| FIM | 12.5% | 6/48 |
| Lymphoma | 20.1% | 10/48 |
| Dysgammaglobulinemia | 56.3% | 27/48 |
| Gut | 8.3% | 4/48 |
| Other | 14.6% | 7/48 |
| EBV status | ||
| EBV+ | 66.6% | 32/48 |
| EBV− | 14.6% | 7/48 |
| Unknown | 18.8% | 9/48 |
| Mortality | 37.5% | 18/48 (4 EBV−) |
| Associated with HLH | 81.3% | 13/16 |
| Associated with FIM | 33.3% | 2/6 |
| Associated with lymphoma | 20% | 2/10 1 had previous HLH and died during chemotherapy; 1 had recurrent lymphoma and many other problems |
| Immunoglobulin replacement | ||
| Yes | 70% | 21/30 |
| No | 23.3% | 7/30 |
| Unknown | 6.7% | 2/30 |
| . | . | Number . |
|---|---|---|
| Age at first symptom | 8 y 8 mo (6 mo-40 y) | |
| Age at death | 7.5 y (1-31 y) | |
| Time from presentation to death | 17.3 mo (1 NK) 9 d-18 y | |
| Time from first symptom (in those patients alive) | 12 y (1 NK) 1-39 y | |
| Presenting symptom | ||
| HLH | 31.3% | 15/48 |
| FIM | 10.4% | 5/48 |
| Lymphoma | 16.7% | 8/48 |
| Dysgammaglobulinemia | 29.2% | 14/48 |
| Other | 12.5% | 6/48 |
| Features | ||
| HLH | 33.3% | 16/48 |
| FIM | 12.5% | 6/48 |
| Lymphoma | 20.1% | 10/48 |
| Dysgammaglobulinemia | 56.3% | 27/48 |
| Gut | 8.3% | 4/48 |
| Other | 14.6% | 7/48 |
| EBV status | ||
| EBV+ | 66.6% | 32/48 |
| EBV− | 14.6% | 7/48 |
| Unknown | 18.8% | 9/48 |
| Mortality | 37.5% | 18/48 (4 EBV−) |
| Associated with HLH | 81.3% | 13/16 |
| Associated with FIM | 33.3% | 2/6 |
| Associated with lymphoma | 20% | 2/10 1 had previous HLH and died during chemotherapy; 1 had recurrent lymphoma and many other problems |
| Immunoglobulin replacement | ||
| Yes | 70% | 21/30 |
| No | 23.3% | 7/30 |
| Unknown | 6.7% | 2/30 |
Presentation was highly variable but as expected included HLH/FIM, dysgammaglobulinemia, and recurrent infection. More unusual presentations included 1 patient with central nervous system vasculitis, intracranial hemorrhage and myocardial fibrosis, and peripheral eosinophilia. The course of XLP1, both temporal and clinical, was extremely variable without any apparent correlation to family history or genetic mutation.
As with transplanted patients the significant mortality associated with HLH is evident in untransplanted patients (81.3%). Presentation or manifestation of HLH (n = 15 and 16, respectively) was associated with a rapid decline and death within 6 weeks, especially in patients less than 5 years of age. Of the 48 patients, 32 did not have manifestations of HLH, and in this group 5 died, thereby giving a survival of 84.4% with a mean follow-up in this group of 11.6 years. For those untransplanted patients who survive, 70% received replacement immunoglobulin therapy, with few suffering from long-term complications. Only 5 patients have recorded complications, including 1 with recurrent infection, 1 with neutropenia, 1 with bronchiectasis, and 2 boys with gastrointestinal disease and growth delay.
Supplemental Table 2 compares the demographics between transplanted and untransplanted patients. No significant differences were seen between the 2 populations other than mortality, which was twice as high in the untransplanted cohort (P < .05). Age of death was lower in transplanted patients and may reflect the more severe course that may have led to the need for HSCT.
Discussion
This report summarizes data on 91 patients from 64 families worldwide with a genetic diagnosis of XLP1 and provides information on outcome with and without allogeneic HSCT using current treatment protocols (summarized in Figure 3). This report is the first large-scale analysis of XLP1 patients since the report by the XLP1 registry in 1995 and has for the first time gathered patients who have confirmed SAP/SH2D1A mutations. Therefore this report represents a genetically homogeneous cohort and avoids possible phenotypic variability through inclusion of other patients with genetic defects such as XIAP/BIRC 4 mutations.
The clinical features of the disease are similar to those reported by the XLP1 Registry, with HLH and FIM remaining the most common and most lethal complication. With the advent of more accessible genetic screening and mutation analysis confirming the diagnosis, more patients have been diagnosed early on the basis of family history and increased awareness of the disease has also led to patients being diagnosed after presentation with immune dysregulation and more unusual presenting features such as vasculitis.
A diagnosis of XLP1 is still a difficult one to make, and it is possible that some patients mistakenly fall under the umbrella of common variable immunodeficiency, although previous genetic screening studies suggest that the incidence of XLP1 patients in common variable immunodeficiency cohorts is low.33 It is also possible that there are older individuals who present in adulthood and have not been identified and included in this study, and this may result in a bias in the method of data collection as the majority of centers approached to contribute data were specialist pediatric centers. For example, a recent case report describes a 41-year-old man who presented with an EBV-induced central nervous system B-cell lymphoma and absent B cells.34 The oldest surviving patient from this cohort presented at the age of 7 years with recurrent infections and hypogammaglobulinemia, but remains well without transplant and is receiving replacement immunoglobulin therapy at 46 years of age.
The prognosis for XLP1 has greatly improved since 1995, when Seemayer et al2 reported an overall survival of 25% survival with 71.4% of patients in this cohort alive at the time of data analysis. Indeed, the mortality in untransplanted patients was lower than we expected, with 62.5% surviving, including 3 boys who presented with HLH, but the mortality in this group secondary to HLH remains high at 81.3%. It is also interesting to note that a considerable mortality of 28.6% is seen in EBV-negative patients who do not receive HSCT and is related to HLH, sepsis, and vasculitis, suggesting that underlying immunological abnormalities in XLP1, and not only EBV-driven disease, can be fatal. Few complications from recurrent infection and immune dysregulation were reported, suggesting that early diagnosis and good supportive care with replacement immunoglobulin and prophylactic antibiotics can improve the outcome for untransplanted patients. Although over 60% of patients survive without HSCT, it will be important to follow patients carefully, since there is the potential for more severe manifestations to arise, and the options for transplant should be explored.
The mortality associated with the different clinical phenotypes has changed over time, with an improved survival for both HLH (34.5% vs 4%) and lymphoma (91% vs 35%).2 This most likely reflects improved treatment strategies for both HLH (especially the use of agreed protocols such as HLH 9435 and 200436 ) and malignancy. Although these figures represent survival with either HLH or lymphoma as features of XLP1 at any stage, they are very similar to the survival seen if patients present with these features (44.5% and 92% for HLH/FIM and lymphoma, respectively). A mortality of 13% in patients who exhibit dysgammaglobulinemia is associated with HLH, infection, vasculitis, and hemorrhage and highlights that although clinically this phenotype may be milder, it is not an innocuous phenotype, and progression to further fatal symptoms is not uncommon.
The outcome data following allogeneic HSCT from this report is encouraging. The outcome data presented is the largest ever gathered and shows that approximately 80% of patients survive the procedure with complete cellular and humoral reconstitution in the large majority of cases. In this series, there is little evidence of problematic EBV reactivation adversely affecting transplant outcome and no increased incidence of long-term complicating features such as autoimmunity in comparison to transplant for other conditions.37,38 Although donor chimerism in the majority of patients was complete, even low level chimerism in 2 patients with 5% and 20% donor chimerism was associated with good immune recovery. Conversely however, when the patients who required ongoing immunoglobulin support are analyzed, all but 1 have 100% donor engraftment. Further detailed lineage-specific analysis and study of T- and B-cell function in these patients is necessary to determine why humoral function has not been established. The availability of a fully matched donor is associated with an improved survival outcome (approximately 92%), although with the present low numbers this is not statistically significant. Haploidentical grafts show a good outcome in this cohort, but the numbers are extremely low (only 4 transplants performed), and therefore this information needs to be interpreted with caution.
The most important factor affecting survival after transplant is a manifestation of HLH, which significantly reduces survival to 50%. Indeed all 8 patients who died had a complication of HLH at some point in their clinical course. This may reflect the effects of HLH itself or HLH chemotherapy and immunosuppression on the transplant process, including increased organ related toxicity and increased susceptibility to pathogens. In comparison to data reported on cohorts of patients undergoing transplant for HLH associated with other gene defects (eg, perforin and munc 13-4)39-41 it appears that the outcome for HLH associated with XLP1 is worse and may relate to the multiple immune deficits associated with SAP deficiency. By contrast all XLP1 patients who had no HLH manifestations (n = 27) survived the HSCT procedure.
These data may now allow more informed recommendations to be made regarding transplantation in XLP1. It is clear from this report that HLH in XLP1 has a very poor prognosis if left untransplanted. Therefore any individual with HLH as a manifestation of XLP1 should undergo allogeneic HSCT.
For patients who are newly diagnosed because of a family history but with no clinical features or for those who present with manifestations other than HLH/FIM, the decision to transplant a relatively well child has been more challenging. An important observation from this report is that all patients (n = 27) who went into transplant without prior HLH survived the procedure in comparison to 84.4% survival for those who are untransplanted and have not manifested with HLH. Since progression to HLH without transplant may occur at a later stage, there is a strong argument to transplant all individuals with a diagnosis of XLP1.
However, there is a counter argument to such a recommendation. As with other immunodeficiencies, the data collected and presented here may not give a complete picture of the natural course of XLP1 and is a historical cohort study conducted before the advent of recent improved therapies. Further, milder patients may also remain undiagnosed having been labeled with a diagnosis of common variable immunodeficiency. It is also the case that HLH is most often seen in younger patients (median age of presentation 3.2 years) and older individuals are less likely to manifest with HLH. There may also be reluctance on the part of families and physicians to undertake a transplant in a well child given that, even in the best-case scenario, there will be a certain mortality associated with any allogeneic transplant procedure.
A more pragmatic recommendation would be to undertake transplant in all patients presenting or manifesting with HLH. Similarly for newly diagnosed or young children without any HLH, if a well-matched donor is available, HSCT should be undertaken, since a manifestation of HLH may be catastrophic or may severely compromise transplant outcome. For older individuals, we would still recommend that HSCT be undertaken, but this decision to transplant should be based on available donor status, wellbeing of the patient, and the attitude of family and physician to the risk of transplant. If HSCT is not undertaken immediately, it is recommended that a donor source is identified and that all patients are followed very carefully in case of disease progression and onset of other manifestations, at which point HSCT could be performed rapidly.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the European Society for Immunodeficiencies registry, which helped to identify patients in the European database. We thank the Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation for initiating the study.
This work was supported by the Wellcome Trust (082159/2/07/2; C.B. is a Clinical Research Fellow).
Wellcome Trust
Authorship
Contribution: C.B. designed the research, collected and acquired data, analyzed the data, and wrote the manuscript; H.B.G. assumed overall responsibility for the research, oversaw analysis, and revised the manuscript; and all authors contributed clinical data and reviewed the manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of Inborn Errors Working Party participants, please see the supplemental Appendix.
Correspondence: Hubert B. Gaspar, Center for Immunodeficiency, Molecular Immunology Unit, Institute of Child Health, 30 Guilford St, London, WC1N 1EH, United Kingdom; e-mail: h.gaspar@ich.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal