Abstract
Although the blood vessel-specific fluorescent transgenic mouse has been an excellent tool to study vasculogenesis and angiogenesis, a lymphatic-specific fluorescent mouse model has not been established to date. Here we report a transgenic animal model that expresses the green fluorescent protein under the promoter of Prox1, a master control gene in lymphatic development. Generated using an approximately 200-kb-long bacterial artificial chromosome harboring the entire Prox1 gene, this Prox1-green fluorescent protein mouse was found to faithfully recapitulate the expression pattern of the Prox1 gene in lymphatic endothelial cells and other Prox1-expressing organs, and enabled us to conveniently visualize detailed structure and morphology of lymphatic vessels and networks throughout development. Our data demonstrate that this novel transgenic mouse can be extremely useful for detection, imaging, and isolation of lymphatic vessels and monitoring wound-associated lymphangiogenesis. Together, this Prox1-green fluorescent protein transgenic mouse will be a great tool for the lymphatic research.
Introduction
The Tie2-promoter-driven green fluorescent protein (GFP) transgenic mouse has been widely used to study vascular morphogenesis, gene expression, isolation, and characterization of endothelial cells and in vivo imaging.1,2 In comparison, a lymphatic-specific fluorescent reporter mouse has not been established. Here we report a bacterial artificial chromosome (BAC)–based transgenic mouse that expresses GFP under the direction of the Prox1 promoter, which is active only in lymphatic, but not blood vascular, endothelial cells,3 and thus allows a convenient fluorescence-based visualization of all lymphatic vessels. This mouse model was originally created as a part of the Gene Expression Nervous System Atlas (GENSAT) BAC-transgenic mouse project, a large-scale effort to generate a library of transgenic mice expressing GFP under the promoter of the central nervous system (CNS) genes to investigate the expression pattern of the CNS genes.4 Although the GFP expression in the CNS of the Prox1-GFP mouse has been extensively studied and annotated by the National Center for Biotechnology Information, the utility of this Prox1-GFP mouse for the vascular research has not been explored despite its potential value. In this report, we performed an extensive investigation of the GFP expression pattern of the Prox1-GFP mouse in lymphatic vessels and present our data demonstrating that this Prox1-GFP mouse could be an extremely useful experimental resource for the vascular research.
Methods
Prox1-GFP BAC transgenic mouse (Tg(Prox1-EGFP)221Gsat/Mmcd, cryo-archived) was purchased from the Mutant Mouse Regional Resource Centers. The founder mouse (FVB/N) has been mated to Crl:CD1(ICR) mice before cryopreservation, and recovered litters are out-bred background (FVB/N-Crl:CD1(ICR)). Sources of antibodies are Prox1 (ReliaTech), LYVE1 (Angiobio), CD31 (Dako Denmark), podoplanin (Developmental Studies Hybridoma Bank), and F4/80 (eBioscience). This study was approved by the University of Southern California Institutional Animal Care and Use Committee. Images were acquired and processed using a 20× objective on a Zeiss microscope and AxioVision Digital Imaging software or Leica M165 FC and LAS Montage Imaging software.
Results and discussion
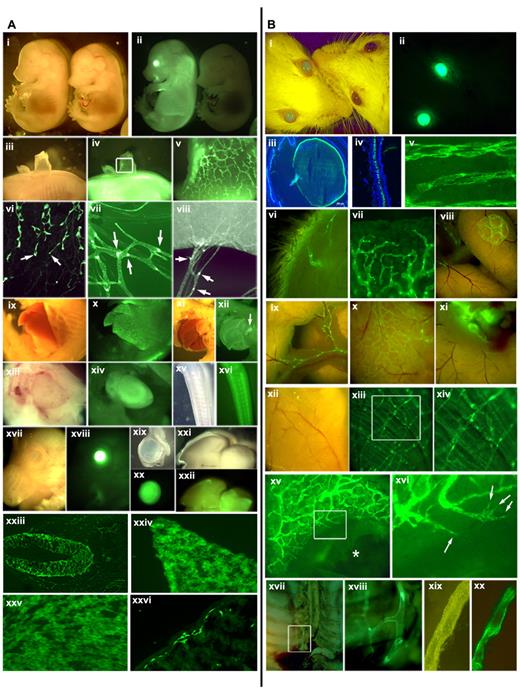
The Prox1-GFP BAC construct was created by inserting the GFP-coding sequences under the Prox1 promoter in a Prox1-harboring BAC (RP23–360I16) through homologous recombination by the GENSAT researchers4 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This BAC contains an approximately 196-kb-long mouse genomic contig, spanning from approximately 115 kb upstream of the Prox1 start codon to approximately 81 kb downstream of the start codon and harbors all regulatory elements that have been proposed to regulate the Prox1 expression, including TCF sites (−48 and −44 kb),5 COUP-TFII site (−10 kb),6 Sox18 (−1 kb),7 and a chromosomal breakpoint for the hypoplastic left heart disease (−78 kb).8 We verified the correct knock-in of GFP in the Prox1 gene based on diagnostic genomic polymerase chain reaction (supplemental Figure 1) and performed extensive analyses of the GFP expression pattern in the Prox1-GFP embryos. Overall morphology of the transgenic embryos appeared normal and indistinguishable from wild-type embryos (E14.5; Figure 1Ai-ii). However, a strong GFP expression was evident in the eye, spinal cord, and liver of Prox1-GFP embryos. Moreover, developing dermal lymphatic plexus and its lymphangiogenic leading front were clearly visible in the dorsal skin of Prox1-GFP embryos (Figure 1Aiii-vi). It has been reported that Prox1 expression is down-regulated in luminal lymphatic endothelial cells (LECs) of the collecting lymphatics but remains high in the intraluminal valves of the collecting lymphatic vessels.9 Consistently, a stronger GFP expression was found in the valves, than luminal LECs, of mesenteric lymphatic vessels (Figure 1Avii-viii). We also investigated GFP expression in other Prox1-expressing organs10-18 and detected prominent GFP signals in the developing liver, pancreas, heart, CNS, eyes, brain, intestine (Figure 1Aix-xxvi) as well as in the lens, retina, and neural tube of Prox1-GFP embryos (supplemental Figure 2). Moreover, we verified that the green fluorescent signals from Prox1-GFP mice were not nonspecific autofluorescence but specifically resulted from the GFP expression by closely comparing wild-type versus transgenic mice (supplemental Figure 3).
GFP expression pattern in the Prox1-GFP BAC transgenic mice. (A) GFP expression in embryos and newborn mice: stereoscopic images of a Prox1-GFP (left) and a wild-type embryo (right) (E14.5) in the bright-field and green fluorescence channel (i-ii). (iii-v) A patch of Prox1-GFP embryonic back skin was peeled to visualize the dermal lymphatic network (v, enlarged image of the boxed area of subpanel iv). (vi) Leading front (arrows) of embryonic dermal lymphatic vessels. (vii-viii) Mesenteric lymphatic vessels and lymphatic valves (arrows) in a newborn mouse (P1). Bright-field and green fluorescent images of the embryonic liver (ix-x), the pancreas (xi-xii), the heart (xiii-xiv), the nervous system in the tail (xv-xvi), the eye (xvii-xviii), isolated eyeball (xix-xx), and the brain (xxi-xxii). Expression of GFP reporter in embryonic neural tube (xxiii), hepatocytes (xxiv), heart muscle (xxv), and intestine (xxvi) of newborn mice. (B) GFP expression in the Prox1-GFP BAC transgenic adult mice: bright-field and green fluorescent imaging allows a convenient distinction of transgenic (left) versus wild-type control (right) mice (i-ii). Cross sections of the eye lens (iii) and the retina (iv). Lymphatic vessels are shown in whole-mount preparations of the trachea (v), inner side of the skin (vi), ear edge (vii), Peyer patch (viii), mesentery (ix), surface of intestine (x) and lymph nodes (xi), diaphragm (xii-xiii), and an enlarged view (xiv) of boxed area in panel xiii. (xv-xvi) The ear of a Prox1-GFP mouse was wounded with an ear punch. *Lymphatic vessel regeneration was visualized at the edge of the wound at day 7. Filopodia of sprouting lymphatic endothelial cells are shown in a boxed area in panel xv and marked with arrows in the enlarged image (xvi). The thoracic duct of a Prox1-GFP mouse can be easily visualized in the retroperitoneal space (xvii) with an enlarged view (xviii). An isolated thoracic duct clearly shows a strong GFP positivity (xix-xx).
GFP expression pattern in the Prox1-GFP BAC transgenic mice. (A) GFP expression in embryos and newborn mice: stereoscopic images of a Prox1-GFP (left) and a wild-type embryo (right) (E14.5) in the bright-field and green fluorescence channel (i-ii). (iii-v) A patch of Prox1-GFP embryonic back skin was peeled to visualize the dermal lymphatic network (v, enlarged image of the boxed area of subpanel iv). (vi) Leading front (arrows) of embryonic dermal lymphatic vessels. (vii-viii) Mesenteric lymphatic vessels and lymphatic valves (arrows) in a newborn mouse (P1). Bright-field and green fluorescent images of the embryonic liver (ix-x), the pancreas (xi-xii), the heart (xiii-xiv), the nervous system in the tail (xv-xvi), the eye (xvii-xviii), isolated eyeball (xix-xx), and the brain (xxi-xxii). Expression of GFP reporter in embryonic neural tube (xxiii), hepatocytes (xxiv), heart muscle (xxv), and intestine (xxvi) of newborn mice. (B) GFP expression in the Prox1-GFP BAC transgenic adult mice: bright-field and green fluorescent imaging allows a convenient distinction of transgenic (left) versus wild-type control (right) mice (i-ii). Cross sections of the eye lens (iii) and the retina (iv). Lymphatic vessels are shown in whole-mount preparations of the trachea (v), inner side of the skin (vi), ear edge (vii), Peyer patch (viii), mesentery (ix), surface of intestine (x) and lymph nodes (xi), diaphragm (xii-xiii), and an enlarged view (xiv) of boxed area in panel xiii. (xv-xvi) The ear of a Prox1-GFP mouse was wounded with an ear punch. *Lymphatic vessel regeneration was visualized at the edge of the wound at day 7. Filopodia of sprouting lymphatic endothelial cells are shown in a boxed area in panel xv and marked with arrows in the enlarged image (xvi). The thoracic duct of a Prox1-GFP mouse can be easily visualized in the retroperitoneal space (xvii) with an enlarged view (xviii). An isolated thoracic duct clearly shows a strong GFP positivity (xix-xx).
We then carried out an in-depth analysis whether the tissue-specific GFP expression maintains in adult Prox1-GFP mice. The transgenic adults are indistinguishable from wild-type littermates in gross morphology and phenotype, except for their green eyes, even under nonfluorescent lightings (Figure 1Bi-ii). This unique phenotype was found to be the result of overexpression of GFP in the lens and retina throughout development (Figure 1Biii-iv) and allowed a convenient identification of the transgenic mice. Moreover, adult lymphatic vessels could be easily detectable in whole-mount preparations of the trachea, skin, ear, intestine, and diaphragm without any immunostainings (Figure 1Bv-xiv). In addition, we introduced puncture wounds into the adult ears and were able to monitor lymphatic sprouting during wound healing (Figure 1Bxv-xvi), suggesting the potential value of this model for in vivo noninvasive real-time imaging of wound- or tumor-associated lymphangiogenesis. Finally, we attempted to isolate the thoracic lymphatic duct, a biggest-caliber lymphatic vessel, which is difficult to isolate because of its hidden anatomic location and elusive morphology without contrasting dye injection. Indeed, we were able to easily locate and isolate the thoracic duct in the thoracic cavity of Prox1-GFP mouse under a fluorescent stereomicroscope (Figure 1Bxvii-xx; supplemental Figure 4).
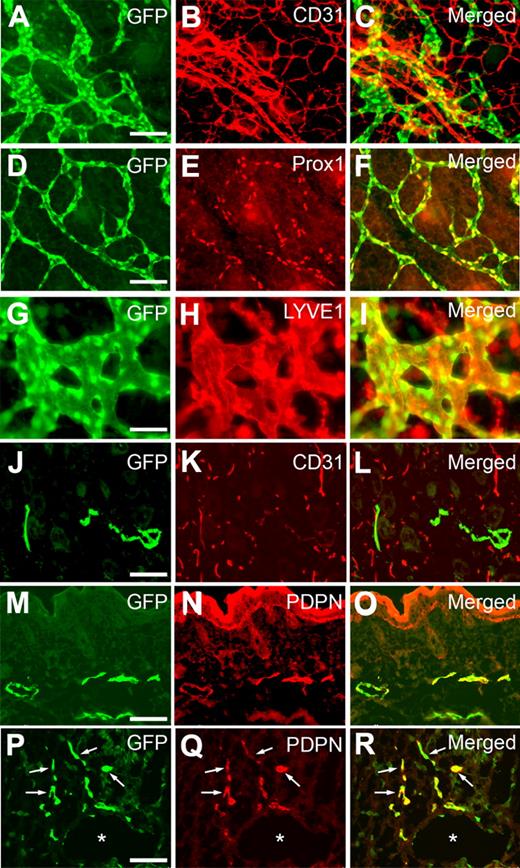
We next performed comparative analyses on GFP expression in the lymphatics of Prox1-GFP transgenic embryos using additional lymphatic-specific markers. Whole-mount staining of embryonic back skins against CD31 showed a GFP expression specifically in LECs, but not in blood vascular endothelial cells (Figure 2A-C), that CD31 primarily marks because of its low expression in LECs.19 Moreover, GFP expression was colocalized with Prox1 expression in embryonic dermal lymphatic network (Figure 2D-F) and also overlapped with the expression of another lymphatic marker LYVE1 in lymphatic vessels (Figure 2G-I). LYVE1-positive cells lacking GFP signal were found to be F4/80-positive macrophages as previously shown20,21 (supplemental Figure 5). In addition, dermal sections of Prox1-GFP newborn pups also clearly demonstrate lymphatic-specific GFP expression based on costaining against CD31 and podoplanin (Figure 2J-O). Finally, we prepared cross sections of the whole embryo at E10.5 to visualize the initial event of lymphatic differentiation of vascular endothelial cells3,22 and were able to detect a set of lymphatically differentiating, GFP(Prox1)/podoplanin-double-positive endothelial cells that migrate from the embryonic cardinal vein (Figure 2P-R; supplemental Figure 6). Together, our study demonstrates that GFP signal in Prox1-GFP transgenic mice presents the endogenous expression pattern of Prox1 and can be successfully used to visualize LECs and lymphatic vessels.
Colocalization of the GFP signal with various lymphatic markers throughout development. Whole-mount staining of dermal lymphatics in Prox1-GFP transgenic embryos (E14.5) against CD31 (A-C), Prox1 (D-F), and LYVE1 (G-I) show that GFP expression is colocalized with the expression of Prox1 and LYVE1. Moreover, staining of skin cross section of newborn pup (P1) against CD31 (J-L) and podoplanin (PDPN, M-O) specifically found GFP signal in lymphatic endothelial cells, suggesting a lymphatic-specific expression of GFP. (P-R) *Podoplanin staining of a cross section of Prox1-GFP embryo at E10.5 shows the budding LECs (arrows) from the embryonic cardinal vein. Scale bars represent 100 μm with an exception (G-I, 50 μm).
Colocalization of the GFP signal with various lymphatic markers throughout development. Whole-mount staining of dermal lymphatics in Prox1-GFP transgenic embryos (E14.5) against CD31 (A-C), Prox1 (D-F), and LYVE1 (G-I) show that GFP expression is colocalized with the expression of Prox1 and LYVE1. Moreover, staining of skin cross section of newborn pup (P1) against CD31 (J-L) and podoplanin (PDPN, M-O) specifically found GFP signal in lymphatic endothelial cells, suggesting a lymphatic-specific expression of GFP. (P-R) *Podoplanin staining of a cross section of Prox1-GFP embryo at E10.5 shows the budding LECs (arrows) from the embryonic cardinal vein. Scale bars represent 100 μm with an exception (G-I, 50 μm).
In the past, attempts have been made to establish a lymphatic-specific fluorescent mouse model using the promoters of VEGFR-3 and podoplanin. However, the 1.6-kb VEGFR-3 promoter23 and the 1.3-kb podoplanin promoter24 failed to recapitulate the expression patterns of their respective genes in transgenic embryos and adults. Alternatively, nuclear factor-κB activity has been fortuitously associated with lymphatic vessels.25 This LacZ reporter-based model, however, has drawbacks of ubiquitous nuclear factor-κB expression in other cell types under various physiologic and pathologic conditions. Despite the creation of the Prox1-GFP transgenic mouse as part of the GENSAT effort many years ago, its experimental value in vascular research has never been explored to date. Our data presented here demonstrate that the approximately 196-kb-long Prox1 genomic DNA in the BAC used for Prox1-GFP mouse harbors all the regulatory DNA elements required for a faithful expression of the GFP reporter in LECs and other Prox1-expressing organs and that this Prox1-GFP mouse model can be potentially used for in vivo vascular imaging, gene expression, cell isolation and characterization, tumor metastasis, immune cell trafficking, and more, serving as a great experimental resource for vascular research.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the American Heart Association, American Cancer Society, March of Dimes Foundation, and National Institutes of Health/National Heart, Lung, and Blood Institute (1R21HL082643, R01HD059762).
National Institutes of Health
Authorship
Contribution: I.C., H.K.C., S.R., H.N.L., K.E.K., S.L., J.Y., D.C., Y.S.L., and B.A. designed and performed experiments and analyzed data; and Y.K.H. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Young-Kwon Hong, Departments of Surgery, and Biochemistry and Molecular Biology, University of Southern California, Norris Comprehensive Cancer Center, 1450 Biggy St, NRT6501, Mail Code 9601, Los Angeles, CA 90033; e-mail: young.hong@usc.edu.
References
Author notes
I.C. and H.K.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal