Prostaglandin E2 (PGE2) is an inflammatory mediator often used to increase CCR7 expression in the dendritic cells (DCs) used as cancer vaccines and to enhance their responsiveness to lymph node–associated chemokines. Here, we show that high surface expression of CCR7 on PGE2-matured DCs is associated with their suppressed production of the endogenous CCR7 ligand, CCL19, and is reversible by exogenous CCL19. In contrast to the PGE2-matured DCs, DCs matured in the presence of toll-like receptor (TLR) ligands and interferons produce high levels of both CCL19 and CCR7 mRNA/protein, but show selectively reduced expression of surface CCR7, which is compensated after DC removal from the CCL19-rich maturation environment. In accordance with these findings, PGE2-matured DCs show significantly higher in vitro migratory responsiveness to lymph node–associated chemokines directly after DC generation, but not after additional short-term culture in vitro, nor in vivo in patients injected with 111indium-labeled DCs. The differences in CCL19-producing ability imprinted during DC maturation result in their different abilities to attract CCR7+ naive T cells. Our data help to explain the impact of PGE2 on CCR7 expression in maturing DCs and demonstrate a novel mechanism of regulatory activity of PGE2, mediated by the inhibition of DCs ability to attract naive T cells.
Introduction
Prostaglandin E2 (PGE2) is an inflammatory mediator with suppressive activity at several levels of the immune response.1,–3 PGE2 selectively impairs the production of interleukin-2 (IL-2) and interferon-γ (IFNγ) in T cells,4,5 inhibits the responsiveness to T cell–activating and Th1-driving cytokines such as IL-2 and IL-12p70,6,7 blocks the production of dendritic cell (DC)-produced proinflammatory cytokines, including IL-12p70,8,–10 and induces the production of IL-12R antagonist IL-12p40 homodimer.11,12 Recently, PGE2 has been also shown to promote the ability of DCs to preferentially attract the inhibitory regulatory T cell (Treg) subset of CD4+ T cells13 and to directly promote the development of Tregs.14,15
In apparent contrast to these suppressive functions, PGE2 also has been reported to synergize with tumor necrosis factor-α (TNFα) in the induction of DC maturation10,16 and in promoting CCR7 expression and the chemotactic responsiveness of DCs to CCL19 and CCL21,17,–19 the 2 CCR7 ligands known to promote DC entry into lymph nodes.20,–22 These observations opened the possibility that PGE2 may also support the induction of antigen-specific immune responses by promoting the migration of Ag-carrying DCs to the draining lymph nodes and their interaction with lymph node–based naive and central memory T cells. Based on these observations, PGE2 is frequently included in the cytokine cocktails16 used to induce mature DCs for clinical use as vaccines against cancer.23
Taking into account these apparently paradoxical effects of PGE2 on DC functions and guided by the observations from other cell systems that the surface levels of chemokine receptors can be regulated by their chemokine ligands,24,,–27 we tested the impact of PGE2 on the regulation of the CCR7-CCL19/21 system in maturing DCs.
Methods
Media and reagents
Serum-free CellGro DC medium (CellGenix) was used for the DC cultures. The following factors were used to generate mature DC: recombinant human (rhu) granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 (gifts from Schering-Plough); IFNα (Intron A-IFN-α-2b; Schering-Plough); rhuTNFα, rhuIL-1β, and rhuIFNγ (Strathmann Biotech); rhuIL-6 (Genzyme); PGE2 and poly-I:C (both from Sigma-Aldrich). CD40L-transfected J558 cells28 (gift from Dr P. Lane, University of Birmingham, United Kingdom) were used as an equivalent to activated CD4+ T cells.28,29 Recombinant CCL19 and CCL21 were purchased from Peprotech. Fluorescein isothiocyanate (FITC)–labeled CCR7-specific antibody for flow cytometry (clone 150503; R&D Systems) and the relevant isotype control (immunoglobulin G2a [IgG2a]) used for fluorescence-activated cell sorting (FACS) staining were obtained from R&D Systems. CCR7 blocking antibody (3D12) was purchased from BD Biosciences.
DC cultures
Peripheral blood mononuclear cells (PBMCs) obtained from healthy donors were isolated with lymphocyte separation medium (CellGro; Mediatech). Monocytes were isolated on density gradients, using Percoll (Sigma-Aldrich) or Isolate (Irving Scientific) followed by plastic adherence as described.29,30 Monocytes were cultured for 6 days in 24-well plates (Falcon; Becton Dickinson Labware) at 5 × 105 cells per well in rhu GM-CSF and IL-4 (both 1000 IU/mL). On day 6, DC maturation (48 hours) was induced using the indicated combinations of the following factors: IL-1β (25 ng/mL), TNFα (50 ng/mL), IFNγ (1000 U/mL), IL-6 (1000 U/mL), PGE2 (10−6 M), poly-I:C (20 μg/mL), and IFNα (3000 U/mL).30
Isolation of naive CD4+ T cells
Mononuclear cells from peripheral blood of healthy donors were isolated on density gradient using Lymphocyte Separation Medium (CellGro). Naive CD4+ T cells were isolated by negative magnetic selection using the EasySep human CD4+ T cell enrichment kit (StemCell Technologies) according to the manufacturer's protocol.
Analysis of CCL19 and CCL21 production by Taqman and ELISA
Total RNA was extracted using the RNAeasy kit (QIAGEN) according to the manufacturer's protocol. cDNA synthesis was performed on 2 μg of extracted RNA in a 20-μL reaction volume using Retroscript kit (Ambion) according to the manufacturer's protocol. Real-time analysis was performed on a 25-ng sample cDNA using premade CCR7-, CCL19-, and CCL21-specific Taqman primers and probes from Applied Biosystems, in 25-μL reaction volume using universal Taqman kit with uracil-N-glycosylase (UNG; Applied Biosystems) following the manufacturer's protocol. Samples were analyzed with an ABI Prism 7700 sequence analyzer (Applied Biosystems). The expression of each gene was normalized to HPRT1 housekeeping gene and expressed as fold increase (2−ΔCT), where ΔCT = [CT(target gene)] − [CT(HPRT1)]. Supernatants from DC cultures (20 × 103 DC/0.2 mL, when indicated stimulated with 50 × 103 CD40L-expressing J558 cells) were analyzed for CCL19 protein concentration by indirect sandwich enzyme-linked immunosorbent assay (ELISA), using primary and secondary antibodies from Peprotech. In brief, ELISA plates (Corning Inc) were coated overnight at room temperature with the primary antibody (10 μg/mL), followed by washing and blocking with phosphate-buffered saline (PBS) plus 4% bovine serum albumin (BSA) for 1 hour. The samples were added to the wells and incubated for 1 hour and subsequently washed and incubated with the biotinylated secondary antibody (2.5 μg/mL) for 1 hour. The plates were washed and incubated for 30 minutes with streptavidin-horseradish peroxidase (HRP) conjugate (Pierce Biotechnology), diluted 1:8000 in wash buffer (50 mM Tris, 0.2% Tween). The plates were washed and detected with 50 μL tetramethylbenzidine (TMB) substrate (Pierce Biotechnology). Reactions were stopped with 2% H2SO4, and absorbance at 450 nm was measured.
Analysis of surface and intracellular CCR7 levels
FITC-labeled CCR7 antibody (clone 150503; R&D Systems) was used for surface and intracellular CCR7 staining. For intracellular staining, the cells were permeabilized with Permiflow (Invirion) reagent and then stained with CCR7 antibody.
Analysis of in vitro chemotaxis
DC chemotaxis assays were performed in 96-transwell plates with 5-μm pore size polycarbonate filter (Corning Inc.). The lower chamber was filled with 200 μL recombinant human CCL21 in DC medium (Cellgenix). DCs (50 × 103) in 50 μL were added to the upper chamber, and the migration chambers were incubated for 2 hours at 37°C. To analyze chemotaxis of naive CD4+ T cells, 5.0-μm pore size 24-well transwell plates from the same company were used. Cells (200 × 103) of naive CD4 T cells (in 100 μL) were allowed to migrate for 3 hours toward DC culture supernatants (250 × 103 DC/0.5 mL, stimulated for 24 hours with 250 × 103 CD40L-expressing J558 cells). When indicated, to block the CCR7-dependent component of migration, naive CD4+ T cells were treated for 30 minutes with anti-CCR7 blocking antibody (3D12, 20 μg/mL; BD Biosciences) before chemotaxis.
Analysis of DC migration in vivo in melanoma patients
The clinical trial was approved by the ethics committee of the University of Heidelberg. Patients with stage IIIc-IV melanoma were eligible. DCs for clinical use were generated under cGMP conditions from autologous monocytes obtained by leukapheresis. Monocytes were selected using CD14 microbeads (CliniMACS; Miltenyi Biotec) and cultured in CellGro DC medium (CellGenix) supplemented with GM-CSF (800 IU/mL; Novartis) and IL-4 (1000 IU/mL; CellGenix) for 6 days. On day 6, maturation was induced by either (for standard DCs [sDCs]) TNFα (5 ng/mL Beromun, a gift from Boehringer-Ingelheim), IL-1β (2 ng/mL; CellGenix), IL-6 (5 ng/mL: CellGenix), and PGE2 (1 μg/mL Dinoproston; Pfizer) or (for αDC1s) a cocktail of TNFα (50 ng/mL), IL-1β (25 ng/mL), IFN-γ 1000 U/mL (Immukin; Boehringer-Ingelheim) IFN-α2a (1000 U/mL, Roferon A; Roche), and poly-I:C (20 μg/mL; Sigma-Aldrich). DCs were labeled with 1.4 MBq 111indium-oxinate (Tyco Healthcare) for 15 minutes and washed with PBS/1% human serum albumin. The labeling efficiency was 71.8% (± 6.4%) with viability of more than 95% at 24 hours after the labeling procedure. One million 111indium-labeled DC were injected intradermally on both upper thighs (αDC1s, right leg; sDCs, left leg) 10 cm distal to the inguinal lymph nodes. Migration was followed by scintigraphic imaging with a single-photon emission computed tomography/computed tomography (SPECT/CT) camera (Hawkeye; GE Healthcare) directly after injection, and 1, 24, and 48 hours after injection. We performed whole body scans images for 20 minutes (10 cm/min) and single planar scans of the pelvis for 10 minutes. Activity that accumulated in lymph nodes was quantified by region of interest analysis.
Statistical analysis
Chemokine dose-responsiveness of DCs in vitro was analyzed by analysis of variance (ANOVA), whereas the in vivo migration was analyzed by Wilcoxon matched-pair test. In all other cases, the data were evaluated using t test (2-tailed), with P values less than .05 considered as significant.
Results
PGE2-matured sDCs and αDC1s show transient differences in CCR7 expression and CCR7 responsiveness in vitro, but similar lymph node–migratory function in vivo
PGE2-matured sDCs and αDC1 are 2 types of mature DCs that can be generated in clinically applicable serum-free conditions. Although the PGE2-based protocols of DC maturation have been shown to be associated with suppression of IL-12p70 production,10,–12,30 the ability of PGE2 to enhance the DC expression of CCR7 and in vitro migratory responsiveness to CCL19 and CCL2117,–19 (CCR7 ligands guiding DCs to lymph nodes20,–22,31 ) led to the application of PGE2 in inducing mature sDCs for clinical applications.16,23 In contrast to such IL-1β/TNFα/IL-6/PGE2–induced sDCs, mature type 1–polarized DCs obtained in the presence of toll-like receptor (TLR) ligands and IFNs,30,32,–34 including IL-1β/TNFα/IFNα/IFNγ/poly-I:C–induced, type 1–polarized DCs; αDC1,30 are capable of producing much higher levels of IL-12 and inducing tumor-specific cytotoxic T lymphocytes (CTLs) in vitro,30,33,35 but show lower in vitro migratory responsiveness to CCR7-binding chemokines.30
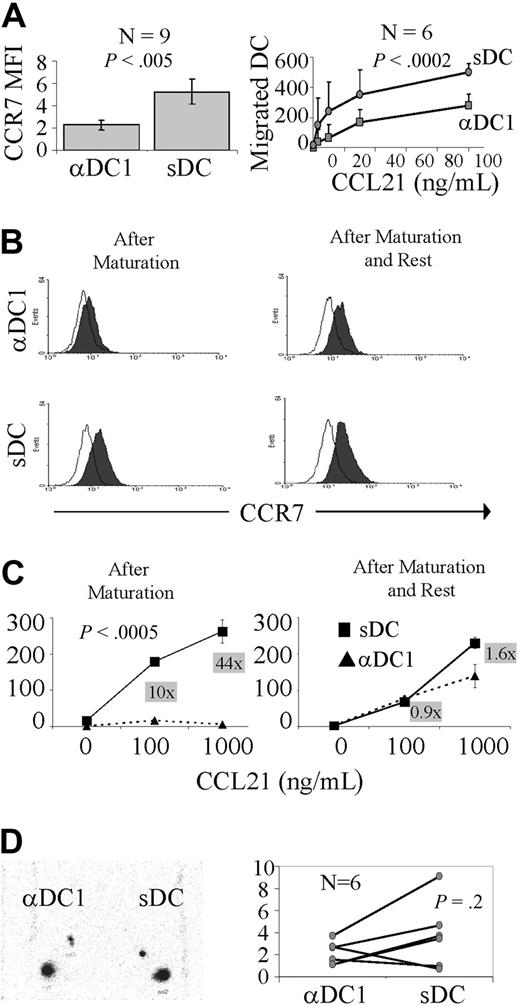
In accordance with previous observations,30,35 PGE2-matured DCs showed statistically higher surface CCR7 expression than αDC1s (Figure 1A left) and elevated ability to migrate toward CCL21, the lymph node–associated CCR7 ligand (Figure 1A right), despite significant variability of the magnitude of such differences observed in DC preparations from different donors. However, even the most profound differences in CCR7 expression on sDCs and αDC1s observed in some donors (Figure 1B left) were rapidly compensated after the harvesting of DCs from the maturation cultures and replating them in neutral conditions in the presence of GM-CSF only (Figure 1B right). This compensation of the differences in surface CCR7 expression was reflected by the elimination of differences in migratory capacity in vitro (Figure 1C).
Transient elevation of CCR7 expression and in vitro migratory responsiveness to lymph node–associated chemokines in PGE2-matured DCs. (A left) Surface CCR7 expression in DCs matured into αDC1s or sDCs in 9 different donors. (A right) In vitro migratory response of αDC1s and sDCs to CCL21, a secondary lymphoid organ chemokine (data from 6 different donors shown as mean ± SD). (B) Surface expression of CCR7 protein on αDC1s and sDCs at 0 (left) and 24 hours after completion of maturation and replating in the absence of the maturation-inducing factors (right). (C) In vitro migratory response to CCL21 of αDC1s and sDCs, directly (left) and 24 hours (right) after removal from the maturation cultures. The numbers in gray boxes represent the ratios between the numbers of sDCs and αDC1 that migrated to each individual concentration of CCL21. Similar data were obtained in 2 independent experiments. (D, left) In vivo migration of αDC1s and sDCs to the lymph nodes. Scintigraphic image of 111indium-labeled αDC1 and sDC at intradermal injection site and draining lymph nodes at 48 hours in a single representative patient. (D, right) In vivo migration of αDC1s or sDCs in 6 patients (each pair of dots represents each individual patient).
Transient elevation of CCR7 expression and in vitro migratory responsiveness to lymph node–associated chemokines in PGE2-matured DCs. (A left) Surface CCR7 expression in DCs matured into αDC1s or sDCs in 9 different donors. (A right) In vitro migratory response of αDC1s and sDCs to CCL21, a secondary lymphoid organ chemokine (data from 6 different donors shown as mean ± SD). (B) Surface expression of CCR7 protein on αDC1s and sDCs at 0 (left) and 24 hours after completion of maturation and replating in the absence of the maturation-inducing factors (right). (C) In vitro migratory response to CCL21 of αDC1s and sDCs, directly (left) and 24 hours (right) after removal from the maturation cultures. The numbers in gray boxes represent the ratios between the numbers of sDCs and αDC1 that migrated to each individual concentration of CCL21. Similar data were obtained in 2 independent experiments. (D, left) In vivo migration of αDC1s and sDCs to the lymph nodes. Scintigraphic image of 111indium-labeled αDC1 and sDC at intradermal injection site and draining lymph nodes at 48 hours in a single representative patient. (D, right) In vivo migration of αDC1s or sDCs in 6 patients (each pair of dots represents each individual patient).
In accord with the ability of the differentially matured DCs to compensate for the maturation-associated differences in CCR7 expression and responsiveness to lymph node–produced CCR7 ligands also in vivo, sDCs and αDC1s showed similar ability to migrate to the draining lymph nodes, following their intradermal injection (Figure 1D; αDC1s, right leg; sDCs, left leg). We observed retention of the radioactivity at the injection site of 79% plus or minus 4% and 55% plus or minus 2% after 24 and 48 hours, respectively, with nodal accumulation starting to be detectable at 24 hours and detectable in all cases at 48 hours. Similar to earlier studies, the overall migratory effectiveness of all DCs was within the range of 0.69% to 4.6% with a single preparation migrating with 9% effectiveness (Figure 1D). In 4 individuals, the migration of sDCs (range, 0.69%-9.1%) was higher than the migration of αDC1s (range, 1.1%-3.7%), whereas αDC1s were superior in 2 of the 6 individuals tested.
Selectively enhanced surface CCR7 expression on PGE2-matured DCs occurs despite reduced levels of CCR7 gene expression and is abrogated by exogenous CCL19
In an attempt to address the mechanism of the transient differences in CCR7 expression between sDCs and αDC1s, we compared surface and intracellular CCR7 protein levels and the expression of CCR7 mRNA. Unexpectedly, even in the donors showing the most pronounced differences in surface CCR7 expression (Figure 2A left), we could not detect such differences when analyzing the total CCR7 protein levels in permeabilized DCs (Figure 2A middle). Even more strikingly, the PGE2-matured sDC, expressed substantially reduced, rather than enhanced, levels of CCR7 mRNA (Figure 2A right).
Selectively elevated CCR7 expression on the surface of PGE2-matured DCs occurs despite reduced levels of CCR7 mRNA and is suppressed by the exposure to CCL19. (A) Directly after harvesting from maturation cultures, αDC1s and sDCs were analyzed for the expression of surface CCR7 (left panel) and total (surface and intracellular; middle panel) CCR7 protein and for CCR7 mRNA expression (right panel). (B) Surface (top) and total (bottom) expression of CCR7 protein was analyzed in αDC1s, and sDCs matured in the absence or presence of exogenous CCL19 (100 ng/mL). Similar data were obtained in 3 independent experiments (3 different donors).
Selectively elevated CCR7 expression on the surface of PGE2-matured DCs occurs despite reduced levels of CCR7 mRNA and is suppressed by the exposure to CCL19. (A) Directly after harvesting from maturation cultures, αDC1s and sDCs were analyzed for the expression of surface CCR7 (left panel) and total (surface and intracellular; middle panel) CCR7 protein and for CCR7 mRNA expression (right panel). (B) Surface (top) and total (bottom) expression of CCR7 protein was analyzed in αDC1s, and sDCs matured in the absence or presence of exogenous CCL19 (100 ng/mL). Similar data were obtained in 3 independent experiments (3 different donors).
Prompted by this dissociation between the levels of surface CCR7 protein and CCR7 mRNA expression and by previous reports showing that surface levels of chemokine receptors can be affected by ligand-induced internalization,24,,–27 we tested whether the differences in surface expression of CCR7 between DCs and αDC1s can be abolished by exogenous CCR7 ligands. In accordance with previous reports,24,,–27 we could not detect any impact of CCL21 on surface CCR7 expression (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, the addition of CCL19, the only DC-produced CCR7 ligand (see below) strongly reduced the surface levels of CCR7 expression of sDCs (Figure 2B top). In contrast to its impact on surface expression of CCR7, CCL19 treatment did not affect the overall levels of CCR7 in sDCs (or αDC1s; Figure 2B bottom).
PGE2-matured DCs show suppressed production of CCL19: PGE2 is a powerful inhibitor of CCL19 production by human DC
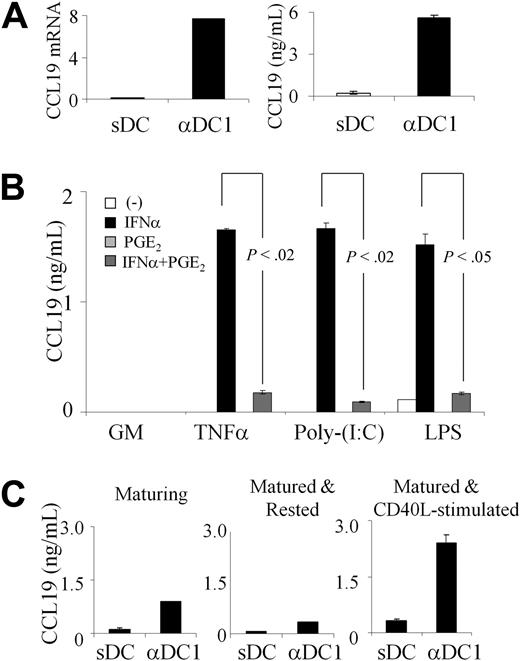
Guided by the selectively enhanced expression of surface CCR7 (but not total cell-associated CCR7 protein or CCR7 mRNA) in PGE2-matured DCs and by the ability of exogenous CCL19 to abrogate that advantage, we compared the ability of PGE2 to affect the production of endogenous CCR7 ligands by human DCs. In accordance with the possibility that the differences in endogenous CCL19 production may affect the rate of internalization of CCR7, we observed high CCL19 protein secretion and high expression of CCL19 mRNA selectively in αDC1s, but not sDC (Figure 3A and supplemental Figure 2). In accordance with the in vivo observations that lymphatic endothelium, rather than DCs, are the source of CCL21,36,37 we did not detect CCL21 expression in either DC type (data not shown).
PGE2 suppresses the production of endogenous CCL19 in DCs induced to mature by TNFα, IFNα, or TLR ligands; poly-I:C or LPS: stability of the maturation-imprinted ability to produce CCL19. (A) CCL19 mRNA (left) and CCL19 protein expression (right) in αDC1s and sDCs. Representative data from one of 6 different donors. (B) CCL19 secretion by DCs exposed for 48 hours to IFNα or PGE2 (or both) in absence or presence of maturation factors, TNFα, poly-I:C (TLR3-ligand), or LPS (TLR4 ligand). Cumulative data (mean ± SEM) from 3 different donors. (C) CCL19 levels in αDC1s or sDCs during maturation or after maturation with or without CD40L. Cumulative data (mean ± SEM) from 3 different donors.
PGE2 suppresses the production of endogenous CCL19 in DCs induced to mature by TNFα, IFNα, or TLR ligands; poly-I:C or LPS: stability of the maturation-imprinted ability to produce CCL19. (A) CCL19 mRNA (left) and CCL19 protein expression (right) in αDC1s and sDCs. Representative data from one of 6 different donors. (B) CCL19 secretion by DCs exposed for 48 hours to IFNα or PGE2 (or both) in absence or presence of maturation factors, TNFα, poly-I:C (TLR3-ligand), or LPS (TLR4 ligand). Cumulative data (mean ± SEM) from 3 different donors. (C) CCL19 levels in αDC1s or sDCs during maturation or after maturation with or without CD40L. Cumulative data (mean ± SEM) from 3 different donors.
Our in-depth analysis of the contribution of the individual DC maturation-inducing factors to the differential regulation of CCL19 production in sDCs and αDC1s, identified TNFα, IFNα, and poly-I:C (TLR3-ligand), as the key CCL19-inducing factors, with PGE2 playing a dominant inhibitory role, suppressing the CCL19 mRNA transcription (supplemental Figure 2) and CCL19 protein secretion (Figure 3B).
PGE2 also showed a dominant inhibitory role, suppressing the CCL19 production induced by another TLR ligand, lipopolysaccharide (LPS; Figure 3B and supplemental Figure 2).
Conditions of maturation determine the ability of mature DCs to produce CCL19 after subsequent (re)activation
To assess the stability of the maturation-induced differences in CCL19 production between the DCs matured in the absence and presence of PGE2, we analyzed the production of this factor during DC maturation (during direct exposure to IFNs and TLR-Ls or PGE2) and after DC removal from these different maturation environments and upon their further culture in the absence or presence of CD40L, used as a model of interaction the differentially matured DCs with CD40L-expressing CD4+ T cells.28,29
We observed that although the difference in CCL19 production between the DCs maturing in the absence or presence of PGE2 was reduced after removal of DCs from the maturation cultures, it was restored after subsequent DC reactivation (Figure 3C). The enhanced CCL19 production in αDC1s was reduced in the DCs harvested and recultured for 24 hours in the absence of maturation-inducing factors, but was restored to similarly high or higher levels after DC harvesting and subsequent CD40L stimulation. In contrast to αDC1s, isolated sDCs failed to produce significant amounts of CCL19 either spontaneously or after subsequent stimulation with CD40L (Figure 3C), despite the absence of PGE2 at this stage. These data indicate that the conditions of DC maturation prime DC for subsequent ability to secrete CCL19 in neutral environments and suggest that DC matured in different conditions may have different abilities to interact with CCL19-sensitive CCR7-expressing T cells (such as naive and central memory T cells) after reaching the draining lymph nodes.
PGE2-matured DC show impaired ability to attract naive T cells
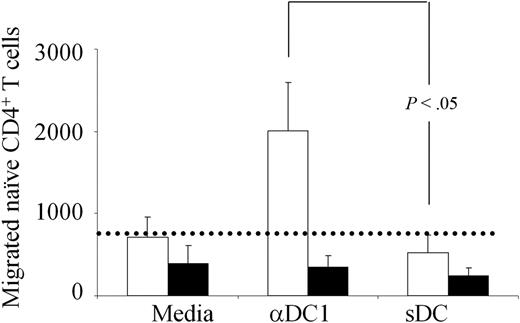
To verify whether the CCL19 produced by αDC1 is indeed functional, we evaluated the ability of αDC1s and sDCs to attract CCR7-expressing naive CD4+ T cells. The contribution of the CCR7 pathway was determined using CCR7 blocking antibodies. As shown in Figure 4, whereas high numbers of naive CD4+ T cells migrated toward αDC1 supernatants, sDCs were completely unable to increase the migration of naive T cells above the background observed with medium alone. As expected, the migration of naive T cells was completely abolished in the presence of CCR7 blocking antibody.
PGE2-matured DCs show suppressed ability to attract naive CD4+ T cells. Negative isolated naive CD4+ T cells were allowed to migrate toward 24-hour culture supernatants from αDC1s and sDCs in transwell migration chambers (3 hours), in the absence (▭) or presence (▬) of CCR7-blocking antibody. The migrated T cells were collected from the bottom chamber and counted. The dotted line represents the average spontaneous migration of T cells in the absence of DC supernatants. Cumulative data (mean ± SEM) from 4 different donors.
PGE2-matured DCs show suppressed ability to attract naive CD4+ T cells. Negative isolated naive CD4+ T cells were allowed to migrate toward 24-hour culture supernatants from αDC1s and sDCs in transwell migration chambers (3 hours), in the absence (▭) or presence (▬) of CCR7-blocking antibody. The migrated T cells were collected from the bottom chamber and counted. The dotted line represents the average spontaneous migration of T cells in the absence of DC supernatants. Cumulative data (mean ± SEM) from 4 different donors.
Discussion
Our data demonstrate a novel function of a chronic inflammatory mediator, PGE2, as a powerful inhibitor of DC production of CCL19, the key chemokine attracting naive and central memory T cells. They show that PGE2 is a dominant inhibitor of the CCL19 production induced by TNFα, IFNs, or TLR-Ls (TLR3 ligand poly-I:C or TLR4 ligand, LPS; see Figure 3B).
The stability of the maturation-induced ability to produce CCL19 indicate that the conditions of DC maturation in peripheral tissues may affect their ability to interact with naive or central memory T cell subsets within the lymph nodes. Although the elevated CCL19 production by the DCs maturing in the presence of TNFα, IFNs, and TLR-Ls (rather than PGE2) is rapidly terminated after DCs leave the presence of IFN- and TLR-L–dominated environment, Ag-carrying DC1 are highly likely to receive secondary chemokine-inducing signals after reaching the lymph nodes from CD40L-expressing Th cells, resulting in a second wave of CCL19 production by IFN- and TLR-L-primed DCs (see Figure 3C) and additional recruitment of naive and central memory T cells.
The current data helps to understand the complex role of PGE2 in the regulation of antigen-specific immune responses and the paradoxical effects of PGE2 on DC functions. PGE2 is an inflammatory mediator overexpressed in multiple inflammatory states, but prevalent at late stages of inflammatory reactions and in chronic inflammation.1,–3 PGE2 was shown to suppress T-cell proliferation and their production of key proinflammatory and antitumor cytokines in direct and indirect manner,4,,,,,,,–12 as well as to promote the interaction of DCs with Tregs13 and to directly promote Treg development.14,15 Paradoxically, however, the ability of PGE2 to promote the in vitro chemotactic responsiveness of DCs to CCL19 and CCL21,17,–19 the 2 CCR7 ligands directing activated DCs into lymph nodes,20,–22 suggested that PGE2 may also support antigen-specific immune responses and led to applications of PGE2-based cytokine cocktails in preparation of DC-based vaccines.
The current results help to reconcile these paradoxical observations and to explain the ability of PGE2 to enhance the expression of CCR7 on the surface of maturing DCs. We observed that the enhanced CCR7 expression on the surface of PGE2-matured DCs occurs despite their reduced, rather than enhanced, expression of CCR7 mRNA, and it is reversed by exogenous CCL19, the CCR7 ligand known to induce CCR7 internalization24,,–27 that is selectively produced by the DCs matured in the absence of PGE2. These data, jointly with the lack of differences at the level of total (surface plus intracellular) CCR7 protein expression, argue that the suppressed production of CCL19, preventing ligand-induced CCR7 internalization, is the key factor responsible for enhanced surface CCR7 expression in DCs maturing in the presence of PGE2.
Importantly, the differences in surface CCR7 expression between the DCs maturing in the absence or presence of PGE2 are rapidly compensated after DC removal from the maturation cultures and after the cessation of high-level production of endogenous CCL19 in DCs matured in the absence of PGE2. The reduced in vitro migratory function of DCs maturing in the absence of PGE2 was clearly evident directly after DC removal from the CCL19-rich maturation cultures, but reduced or eliminated after additional culture of DCs in the absence of CCL19 (Figure 3). In accordance with these observations, our current in vivo data (Figure 1D) also did not demonstrate an advantage of PGE2-matured DCs in lymph node homing. However, they do not preclude the possibility that such an advantage can be demonstrated in larger studies. In either case, our current observations suggest that any potential advantage of PGE2 in promoting DC migration needs to be balanced against its negative impact on the CCL19 production by DCs.
The ability of PGE2, the predominant inflammatory mediator during late stages of inflammation, to program local DCs for preferential interaction with Tregs with the concomitant inhibition of their ability to attract type 1 immune effector cells13 and naive and central memory T cells (the current data) helps to explain the dominance of the suppressive effects at later stage of immune responses and the state of generalized immunosuppression associated with chronic inflammation and cancer, facilitating the design of effective therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Walter Mier for help with the injection of radiolabeled DC, Antje Sucker and Kirsten Kunze for excellent technical support, and William Gooding for statistical support.
This work was supported by grants from the National Cancer Institute (NCI; CA132714, CA134633, and CA121973 to P.K.) and from Harry Lloyd Charitable Trust (to D.S.).
National Institutes of Health
Authorship
Contribution: R.M. designed the laboratory studies, performed the experiments, analyzed the data, and wrote the manuscript; J.M.-B. designed the clinical studies and analyzed the data; U.H. designed and supervised the clinical studies; T.A.R. helped to design the studies, analyze the data, and participated in preparation of the manuscript; D.S. designed the studies, supervised the performance and data analysis of clinical aspects, and wrote the manuscript; and P.K. designed the studies, supervised the performance and data analysis of the laboratory aspects, and wrote the manuscript.
Conflict-of-interest disclosure: α-Type-1 polarized DCs (αDC1s)30 used in this paper are a topic of a pending patent application. None of the authors receives any form of support or remuneration related to αDC1s or other aspects of this study. The authors declare no other competing financial interests.
Correspondence: Pawel Kalinski, Department of Surgery, University of Pittsburgh, Hillman Cancer Center, UPCI Research Pavilion, Rm 1.46b, 5117 Center Ave, Pittsburgh, PA 15213-1863; e-mail: KalinskiP@upmc.edu; or Dirk Schadendorf, Klinik für Dermatologie, Venerologie und Allergologie, Universitätsklinikum Essen, Hufelandstr 55, 45122 Essen, Germany; e-mail: Dirk.Schadendorf@uk-essen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal