The molecular basis for the unique proliferative and self-renewal properties that hierarchically distinguish human stem cells from progenitors and terminally differentiated cells remains largely unknown. We report a role for the Bcl-2 family member myeloid cell leukemia-1 (Mcl-1) as an indispensable regulator of self-renewal in human stem cells and show that a functional dependence on Mcl-1 defines the human stem cell hierarchy. In vivo pharmacologic targeting of the Bcl-2 family members in human hematopoietic stem cells (HSCs) and human leukemic stem cells reduced stem cell regenerative and self-renewal function. Subsequent protein expression studies showed that, among the Bcl-2 family members, only Mcl-1 was up-regulated exclusively in the human HSC fraction on in vivo regeneration of hematopoiesis. Short hairpin RNA–knockdown of Mcl-1 in human cord blood cells did not affect survival in the HSC or hematopoietic progenitor cell fractions in vitro but specifically reduced the in vivo self-renewal function of human HSCs. Moreover, knockdown of Mcl-1 in ontogenetically primitive human pluripotent stem cells resulted in almost complete ablation of stem cell self-renewal function. Our findings show that Mcl-1 is an essential regulator of stem cell self-renewal in humans and therefore represents an axis for therapeutic interventions.
Introduction
Human stem cells are defined by their vast self-renewal and multilineage differentiation capacities, which place them at the top of a cellular hierarchy within a given tissue. Self-renewing multipotent hematopoietic stem cells (HSCs) are at the apex of the hematopoietic hierarchy on the basis of their ability to give rise to all cell types of the hematopoietic system.1 HSCs are followed by a series of hematopoietic progenitor cells (HPCs) with increasingly limited differentiation and self-renewal potential and finally by terminally differentiated mature hematopoietic cells. The hierarchical arrangement found in normal hematopoiesis is conserved in human hematopoietic malignancies such as acute myelogenous leukemia (AML), where rare self-renewing leukemic stem cells (LSCs) capable of initiating leukemogenesis can be detected in the bone marrow (BM) and peripheral blood of some patients.2,3
Independent of their tissue of origin, human stem cells can be more broadly organized into a hierarchy on the basis of their ontogenetic origin. Human pluripotent stem cells (hPSCs) isolated from the inner cell mass of a human blastocyst can be cultured in vitro and are characterized by their ability to expand robustly while maintaining pluripotency or the ability to give rise to all 3 tissue germ layers.4 This property places hPSCs at the top of the human stem cell hierarchy, followed by multipotent adult stem cells such as HSCs that possess more limited tissue-specific differentiation potential. hPSCs, therefore, not only represent a model system for normal hPSC function but also provide a means to study how complex processes such as survival, self-renewal, and differentiation are fundamentally regulated in the most primitive human stem cells.
One of the foremost challenges in human regenerative medicine has been to delineate the molecular basis for the unique self-renewal and differentiation properties that functionally define the human stem cell hierarchy. Human HSC transplantations have been used clinically for more than 40 years5 ; however, the engraftment efficiency of these transplantations is limited by the numbers of donor HSCs in the graft that correlate with hematopoietic reconstitution in the recipient.6 In an attempt to improve HSC transplantation efficiency, human regenerative medicine has focused on selectively increasing the number of donor HSCs by ex vivo expansion by augmenting self-renewal while suppressing differentiation.7,,,,,–13 This has led to the identification of hematopoietic cytokines such as fms-like tyrosine kinase 3 ligand, stem cell factor, and thrombopoietin, as well as the Hedgehog, Notch, and Wnt morphogenic signaling pathways as key regulators of self-renewal and differentiation in human HSCs.14 Nonetheless, efforts to maintain and expand human HSCs in ex vivo culture have been met with limited success,8,11,15,16 and the search for molecular pathways that govern the unique functional properties of human stem cells remains central to the field of regenerative medicine. Although it has been known for almost 20 years that Bcl-2 family members are regulators of critical cell fate decisions such as survival17 and transformation18 in human cell lines, little is known about the roles of individual Bcl-2 family proteins in human normal or cancer stem cells (CSCs).17,19 Previous studies in the mouse hematopoietic system have shown that overexpression of Bcl-2 can augment mouse HSC survival and regenerative capacity20,–22 and that the Bcl-2 homologue myeloid cell leukemia-1 (Mcl-1) is an essential regulator of survival in the primitive lineage-depleted (lin−) c-Kit+ Sca-1+ (LSK) population, which includes both mouse HSCs and HPCs.23 Although these studies positioned the Bcl-2 family members as attractive candidate regulators of survival in the human stem cell fraction, the biologic functions of individual Bcl-2 family proteins have yet to be defined in primary human stem cells.
Accordingly, we examined the role of the Bcl-2 family members as regulators of survival, self-renewal, and differentiation in human stem cells with the use of available in vivo and in vitro assays for stem cell and progenitor function. We describe a role for the Bcl-2 family member Mcl-1 as an indispensable regulator of self-renewal in both human HSCs and hPSCs. Furthermore, in contrast to the mouse system, Mcl-1 dependence was specific to the human stem cell fraction, because the human HPC fraction did not require Mcl-1 for survival or differentiation. Our findings show a tissue-specific and ontogenetic hierarchical dependence of primary human stem cells on Mcl-1 for their self-renewal capacity, indicating that a functional dependence on Mcl-1 for self-renewal is a defining characteristic of human stem cells.
Methods
Mice
For mouse HSC experiments, B6;129-MCL1tm3Sjk/J Mcl-1 homozygous floxed (f/f) mice23,24 (The Jackson Laboratory) and C57BL/6 wild type mice (The Jackson laboratory) were used. For human SCID (severe combined immunodeficient) repopulating cell (SRC) and SCID leukemia-initiating cell (SL-IC) experiments, we used nonobese diabetic (NOD)/Prkdcscid (NOD/SCID)25 and β2 microglobulin knockout (NOD/SCID/B2null)26 mice. Mice were bred and maintained in the human Stem Cell and Cancer Research Institute animal barrier facility at McMaster University. All animal procedures received the approval of the animal ethics board at McMaster University.
Obatoclax
Purification of primitive mouse hematopoietic cells
Mouse BM cells were isolated from the iliac crests, tibiae, and femurs of wild-type and Mcl-1f/f mice. Lin− cells were purified with the use of a StemSep negative selection mouse hematopoietic progenitor enrichment kit (StemCell Technologies).
Purification of primitive human hematopoietic cells
Human umbilical cord blood (CB) mononuclear cells (MNCs) were isolated as described previously.13 Lin− cells were purified from whole MNC populations by negative selection with the use of a custom antibody cocktail kit (StemCell Technologies), whereas CD45+CD34+ cells were prepared by magnetic bead enrichment with the use of either positive or negative selection (StemCell Technologies). MNCs were harvested from the peripheral blood of adult patients with AML as described,13 using centrifugation on Ficoll-Paque. All patient samples were obtained with the approval of local human subject research ethics boards at McMaster University and the University of Western Ontario.
Flow cytometry and cell sorting
Flow cytometry was performed on a FACSCalibur flow cytometer (Becton Dickinson). Cell sorting was performed with a FACSAria cell sorter (Becton Dickinson).
Quantitative intracellular protein staining
Human MNCs and lin− cells were fixed and permeabilized with the Fix and Perm Kit for intracellular flow cytometry (Invitrogen). Cells were first stained for human CD34, CD38, or CD45 for human grafts and then stained with either mouse anti–Bcl-2 fluorescein isothiocyanate (FITC; Becton Dickinson, product no. 340575), rabbit anti–Bcl-xL Alexa Fluor488 (Cell Signaling Technology; product no. 2767), or unconjugated rabbit anti–Mcl-1 (Abcam; product no. ab32087). For isotype controls, cells were stained with either mouse immunoglobulin G (IgG) FITC (Becton Dickinson), rabbit IgG Alexa Fluor 488 (Cell Signaling Technology), or unconjugated rabbit IgG (Abcam). Cells stained with either anti–Mcl-1 or rabbit IgG antibodies were subsequently stained with an anti–rabbit secondary antibody conjugated to either FITC (Jackson ImmunoResearch Laboratories Inc) or Alexa Fluor 647 (Molecular Probes, Invitrogen). Protein expression was assessed quantitatively with the use of the mean fluorescence intensity of the protein signal relative to that of the relevant isotype control.29
qRT-PCR
For quantitative real-time polymerase chain reaction (qRT-PCR), total RNA was isolated with the use of an RNeasy RNA micro isolation kit (QIAGEN). Human lin− cells transduced with lentivirus were sorted to isolate the green fluorescent protein positive (GFP+) fraction before RNA extraction. First-strand cDNA synthesis was performed with the use of a SuperScript III cDNA synthesis kit (Invitrogen). qRT-PCR was performed with the use of the SYBR Green qPCR detection system (Invitrogen) in conjunction with an Mx4000P light cycler (Stratagene). Quantification of transcripts was assessed with the housekeeping gene Gapdh. For genomic PCR, genomic DNA was isolated from sorted GFP+ wild-type and Mcl-1f/f mouse lin− BM cells with the use of a DNeasy Blood and Tissue Kit (QIAGEN). Amplified product sizes were verified on a 1% to 3% (wt/vol) agarose gel stained with ethidium bromide. A complete list of all primer sequences used30 can be found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Lentiviral vectors, production and infection
We targeted Mcl-1 in human lin− cells with the use of lenti-lox 3.7 (pLL3.7).31 pLL3.7 was obtained from Addgene. The short hairpin RNA (shRNA) sequence used to target human Mcl-1 has been described previously32 and corresponds to the following sequence on the human Mcl-1 transcript (National Center for Biotechnology Information accession no. NM_021960): 5′-GCAAGAGGATTATGGCTAA-3′. For knockout of Mcl-1, mouse lin− cells were targeted with pLV-CIG.33 Lentiviral particles were produced with the use of the Virapower packaging system (Invitrogen) with the 293FT packaging cell line (Invitrogen), and high-titer lentiviral stocks were prepared with the use of ultracentrifugation. All lentiviral transductions were performed at a multiplicity of infection of 10 to 100 as described previously.1 Mouse lin− cells were transduced for 24 hours in StemPro 34 medium supplemented with 2mM l-glutamine, 1% bovine serum albumin, 10 ng/mL mouse stem cell factor, and 100 ng/mL mouse thrombopoietin. For hPSC cultures, cell populations were transduced starting at day 2 after passage for 48 hours, in mouse embryonic fibroblast conditioned medium. Gene transfer efficiency was assessed in all cases by flow cytometry.
In vivo hematopoietic repopulation assays
Human and mouse lin− cells and human AML MNCs were transplanted into sublethally irradiated (350-365 cGy, 137Cs) NOD/SCID or NOD/SCID/B2null recipient mice by intravenous tail vein injection and analyzed at 6 to 8 weeks after transplantation for human or mouse donor engraftment as described previously.12 In all secondary transplantations performed, at least 5000 CD34+CD38− cells isolated from primary recipients were injected.
Cell culture
hPSC cultures were maintained on matrigel in mouse embryonic fibroblast conditioned medium as previously described,4 with the use of the hPSC lines H1 ad H9. Cell cultures were passaged every 5 to 7 days, and cultures were analyzed at each passage for total viable, GFP+, and primitive stage-specific embryonic antigen-3+ (SSEA-3+) cells with the use of flow cytometry. Colony-initiating cell assays were performed on sorted GFP+ cells as previously described after passage 1.4 Human lin− CB cells were cultured in serum-free medium (X-VIVO 10) supplemented with hematopoietic cytokines as described.34
Cell viability
Cell viability was assessed with the viability dye 7-amino-actinomycin D (7AAD) or annexin V (Becton Dickinson).
Hematopoietic progenitor assays
Clonogenic hematopoietic progenitor assays for mouse or human HPCs were performed with Methocult H3434 or H4434 (StemCell Technologies) as described previously.12
Image acquisition, data, and statistical analysis
Phase contrast images were acquired with a CoolSNAP digital camera and an inverted microscope (IX51; Olympus). Live cell images shown were analyzed using the software Image-Pro Plus 6.0 (MediaCybernetics) in total 40× magnification (4× objective and 10× optic zoom of the camera). No special imaging solutions were used, as cells were imaged live in culture. Flow cytometric data were analyzed with FlowJo software Version 8.8.7 (TreeStar). qRT-PCR data were analyzed using the MxPro software Version 2.0 (Stratagene) and the ΔΔCt method. The significance of any differences between groups was assessed using the Student t test, and P values less than .05 were considered to be statistically significant.
Results
In vivo pharmacologic inhibition of the Bcl-2 family members leads to decreased human HSC and LSC regenerative function
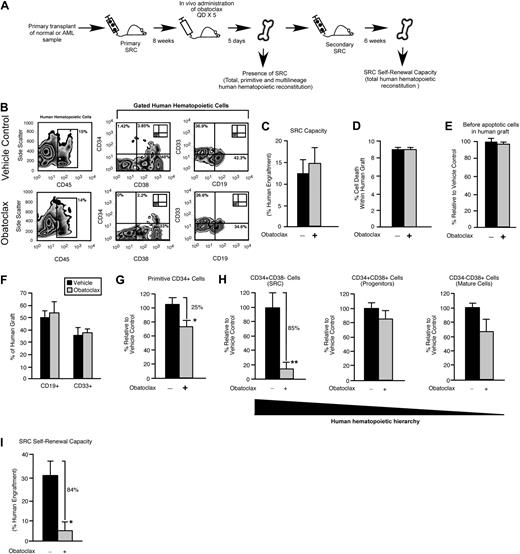
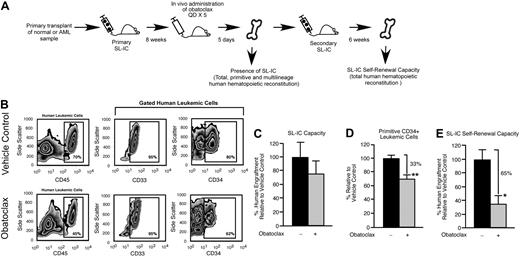
The immune-deficient NOD/SCID mouse provides an in vivo model for the study of both normal and transformed human stem cells. Human HSCs are defined functionally in this xenograft model as SRCs,25 which represent extremely rare cells that uniquely possess the ability to reconstitute human hematopoiesis in NOD/SCID recipients. With the use of the same xenograft model, rare cells that have undergone neoplastic transformation in patients with AML can initiate leukemic grafts and are functionally defined as SL-ICs.35 We used an in vivo strategy to examine whether survival and self-renewal are regulated in human SRCs and SL-ICs by the Bcl-2 family members (Figure 1A). Bcl-2, Bcl-XL, and Mcl-1 were targeted with the use of a small-molecule inhibitor of Bcl-2 family proteins known as obatoclax.27,28 Obatoclax can inhibit the ability of antiapoptotic Bcl-2 family members to bind and sequester proapoptotic Bcl-2 family members, causing increased cell death through apoptosis.27,36 Obatoclax is unique to other Bcl-2 family inhibitor compounds in that it is capable of antagonizing Mcl-1 in addition to Bcl-2 and Bcl-XL.27 In mice reconstituted with human SRCs, administration of obatoclax did not affect total human engraftment (Figure 1B-C), the frequency of lymphoid or myeloid lineage differentiation within the human graft (Figure 1B,F), and did not detectably affect viability or the frequency of preapoptotic cells within the human graft (Figure 1D-E). In addition, administration of obatoclax did not affect the body weight, health, or survival of the recipients compared with vehicle control. We did, however, observe that recipient mice administered obatoclax showed a reduced frequency of primitive human CD34+ hematopoietic cells compared with vehicle control (Figure 1G). On examination of the different populations within the human HSC hierarchy, it was evident that loss of the primitive CD34+ phenotype corresponded to a reduced frequency of CD34+CD38− cells, a population that is enriched for human SRCs37 (18 mice that received a transplant with SRCs isolated from 3 independent human donors; Figure 1H). In contrast, the frequency of cells in the CD34+CD38+ and CD34−CD38+ populations, which are enriched for progenitors and mature cells,37 was unaffected (Figure 1H). To relate this phenotypic observation to SRC function, we performed secondary SRC transplantations as a functional measure of self-renewal and found that administration of obatoclax to primary NOD/SCID recipients caused a greater than 6-fold reduction in the ability of human SRCs to generate secondary hematopoietic grafts (Figure 1I). Similarly, when mice reconstituted by human SL-ICs were administered obatoclax, we observed a reduction in the frequency of the primitive SL-IC–enriched CD34+ hematopoietic cell population within the leukemic graft,35 whereas no change in total leukemic engraftment was observed (18 mice that received a transplant with SL-ICs isolated from 3 independent patients with AML representing French-American-British subtypes M2 and M4; Figure 2A-D). This decrease in primitive phenotype translated into an approximately 3-fold reduction in the ability of SL-ICs to generate secondary leukemic grafts (Figure 2E). Together, these findings identified the Bcl-2 family members as candidate regulators of survival and self-renewal in both normal SRCs and transformed SL-ICs.
In vivo pharmacologic inhibition of the Bcl-2 family members leads to decreased human HSC regenerative and self-renewal function. (A) Experimental strategy used to examine the effect of obatoclax on primitive human hematopoietic cell phenotype and SRC self-renewal capacity. (B) Representative examples of flow cytometric analyses used to assess the effects of obatoclax on human hematopoietic engraftment relative to vehicle control. The inlay plots represent the isotype staining control for each antibody. Percentages represent the frequency of total cells acquired with the indicated cell surface phenotype. (C) Analysis of the effect of obatoclax on human SRC regenerative capacity. The average frequency of human hematopoietic engraftment (CD45+ cells) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (D) Analysis of the effect of obatoclax on the viability of engrafted human hematopoietic cells. The average frequency of dead cells within the human hematopoietic graft (CD45+7AAD+) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (E) Analysis of the effect of obatoclax on apoptosis in engrafted human hematopoietic cells. Average frequency of viable (7AAD−) annexin V+ (preapoptotic) cells within the human hematopoietic graft (CD45+7AAD+) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± the SEM (n = 2-3 mice per group). (F) Analysis of the effect of obatoclax on multilineage human hematopoietic engraftment. The average frequency of lymphoid (CD19+) and myeloid (CD33+) cells within the human hematopoietic graft (gated CD45+ cells) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (G) Analysis of the effect of obatoclax on primitive (CD34+) human hematopoietic engraftment. The average frequency of CD34+ cells within the human hematopoietic graft (gated CD45+ cells) in primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. *P < .05. (H) Analysis of the effect of obatoclax on each population within the human hematopoietic hierarchy. The average frequency of cells in each population within human hematopoietic graft (gated CD45+ cells) in primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. **P < .01. (I) Analysis of the effect of obatoclax on human SRC self-renewal capacity. The average frequency of human hematopoietic engraftment (CD45+ cells) in the BM of secondary recipient mice that received a transplant with human-engrafted BM isolated from primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. *P < .05.
In vivo pharmacologic inhibition of the Bcl-2 family members leads to decreased human HSC regenerative and self-renewal function. (A) Experimental strategy used to examine the effect of obatoclax on primitive human hematopoietic cell phenotype and SRC self-renewal capacity. (B) Representative examples of flow cytometric analyses used to assess the effects of obatoclax on human hematopoietic engraftment relative to vehicle control. The inlay plots represent the isotype staining control for each antibody. Percentages represent the frequency of total cells acquired with the indicated cell surface phenotype. (C) Analysis of the effect of obatoclax on human SRC regenerative capacity. The average frequency of human hematopoietic engraftment (CD45+ cells) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (D) Analysis of the effect of obatoclax on the viability of engrafted human hematopoietic cells. The average frequency of dead cells within the human hematopoietic graft (CD45+7AAD+) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (E) Analysis of the effect of obatoclax on apoptosis in engrafted human hematopoietic cells. Average frequency of viable (7AAD−) annexin V+ (preapoptotic) cells within the human hematopoietic graft (CD45+7AAD+) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± the SEM (n = 2-3 mice per group). (F) Analysis of the effect of obatoclax on multilineage human hematopoietic engraftment. The average frequency of lymphoid (CD19+) and myeloid (CD33+) cells within the human hematopoietic graft (gated CD45+ cells) in the BM of primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (G) Analysis of the effect of obatoclax on primitive (CD34+) human hematopoietic engraftment. The average frequency of CD34+ cells within the human hematopoietic graft (gated CD45+ cells) in primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. *P < .05. (H) Analysis of the effect of obatoclax on each population within the human hematopoietic hierarchy. The average frequency of cells in each population within human hematopoietic graft (gated CD45+ cells) in primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. **P < .01. (I) Analysis of the effect of obatoclax on human SRC self-renewal capacity. The average frequency of human hematopoietic engraftment (CD45+ cells) in the BM of secondary recipient mice that received a transplant with human-engrafted BM isolated from primary recipient mice administered obatoclax or vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. *P < .05.
In vivo pharmacologic inhibition of the Bcl-2 family members leads to decreased human LSC regenerative and self-renewal function. (A) Experimental strategy used to examine the effects of obatoclax on primitive human leukemic cell phenotype and SL-IC self-renewal capacity. (B) Representative examples of flow cytometry analyses used to assess the effects of obatoclax on human leukemic engraftment relative to vehicle control. The BM of recipient mice that received a transplant with human SL-IC and subsequently administered obatoclax or vehicle control was analyzed for total (CD45 expression), myeloid (CD33 expression), and primitive (CD34 expression) human leukemic engraftment. Percentages of 70% and 45% represent the frequency of cells gated for human component in chimeric mice transplanted with pretreated and treated mice, respectively. (C) Analysis of the effect of obatoclax on human SL-IC capacity. The average frequency of human leukemic engraftment (CD45+ cells) in the BM of primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (D) Analysis of the effect of obatoclax on primitive (CD34+) human leukemic engraftment. The average frequency of CD34+ cells within the human leukemic graft (gated CD45+ cells) in the BM of primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. **P < .01. (E) Analysis of the effect of obatoclax on human SL-IC self-renewal capacity. The average frequency of human leukemic engraftment (CD45+ cells) in the BM of secondary recipient mice that received a transplant with human leukemia-engrafted BM isolated from primary recipient mice administered obatoclax is expressed relative to vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. *P < .05.
In vivo pharmacologic inhibition of the Bcl-2 family members leads to decreased human LSC regenerative and self-renewal function. (A) Experimental strategy used to examine the effects of obatoclax on primitive human leukemic cell phenotype and SL-IC self-renewal capacity. (B) Representative examples of flow cytometry analyses used to assess the effects of obatoclax on human leukemic engraftment relative to vehicle control. The BM of recipient mice that received a transplant with human SL-IC and subsequently administered obatoclax or vehicle control was analyzed for total (CD45 expression), myeloid (CD33 expression), and primitive (CD34 expression) human leukemic engraftment. Percentages of 70% and 45% represent the frequency of cells gated for human component in chimeric mice transplanted with pretreated and treated mice, respectively. (C) Analysis of the effect of obatoclax on human SL-IC capacity. The average frequency of human leukemic engraftment (CD45+ cells) in the BM of primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (D) Analysis of the effect of obatoclax on primitive (CD34+) human leukemic engraftment. The average frequency of CD34+ cells within the human leukemic graft (gated CD45+ cells) in the BM of primary recipient mice administered obatoclax is expressed relative to mice administered vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. **P < .01. (E) Analysis of the effect of obatoclax on human SL-IC self-renewal capacity. The average frequency of human leukemic engraftment (CD45+ cells) in the BM of secondary recipient mice that received a transplant with human leukemia-engrafted BM isolated from primary recipient mice administered obatoclax is expressed relative to vehicle control. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. *P < .05.
Mcl-1 expression is uniquely up-regulated in the HSC fraction on in vivo regeneration of human hematopoiesis
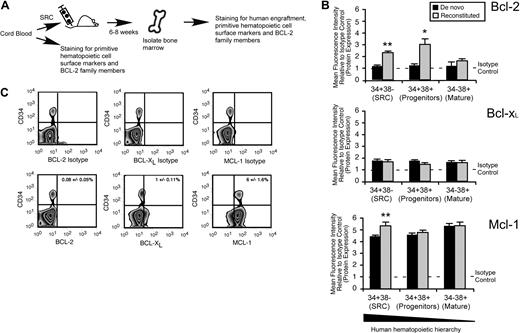
To characterize which Bcl-2 family members may be required for the in vivo function of human SRCs, we used quantitative intracellular staining29,38,–40 to examine expression of Bcl-2, Bcl-XL, and Mcl-1 at the protein level in human CB hematopoietic cells (Figure 3A). With the use of de novo isolated CB hematopoietic cells, we found that Bcl-2 and Bcl-xL were expressed at low to undetectable levels, whereas Mcl-1 was highly expressed in all populations comprising the human hematopoietic hierarchy (Figure 3B). Furthermore, almost all human primitive CD34+ cells also expressed Mcl-1, as opposed to Bcl-XL and Bcl-2 (Figure 3C; supplemental Figure 1B). On in vivo reconstitution of human hematopoietic cells, only Mcl-1 was up-regulated exclusively in the SRC-enriched CD34+CD38− fraction but not in the HPC or mature hematopoietic cell fractions (Figure 3B). Consistent with these findings, Mcl-1 was also up-regulated at the transcriptional level in the primitive CD45+CD34+ fraction of reconstituted human CB cells (supplemental Figure 1C). This indicated a unique role for Mcl-1 specifically in the HSC fraction of the human hematopoietic hierarchy during in vivo regeneration of hematopoiesis.
Mcl-1 is uniquely up-regulated in the HSC fraction of reconstituted human hematopoietic cells. (A) Experimental strategy used to examine the protein expression of Bcl-2 family members in de novo isolated and reconstituted human hematopoietic cells. (B) Quantitative flow cytometric analysis of Bcl-2 family member protein expression in each population within the human hematopoietic hierarchy in both de novo isolated and in vivo reconstituted human hematopoietic cells. The average protein expression of Bcl-2, Bcl-xL, and Mcl-1 in each population of the hematopoietic hierarchy relative to isotype control is shown. Protein expression is the mean fluorescence intensity (MFI) relative to the isotype control. Error bars represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .01. (C) Representative examples of flow cytometry analysis used to assess the frequency of primitive CD34+ human hematopoietic cells that express Bcl-2, Bcl-xL, and Mcl-1. Frequencies represent the percentage of cells positive for the respective protein and CD34 expression ± SEM. Plots are representative of at least 3 independent experiments.
Mcl-1 is uniquely up-regulated in the HSC fraction of reconstituted human hematopoietic cells. (A) Experimental strategy used to examine the protein expression of Bcl-2 family members in de novo isolated and reconstituted human hematopoietic cells. (B) Quantitative flow cytometric analysis of Bcl-2 family member protein expression in each population within the human hematopoietic hierarchy in both de novo isolated and in vivo reconstituted human hematopoietic cells. The average protein expression of Bcl-2, Bcl-xL, and Mcl-1 in each population of the hematopoietic hierarchy relative to isotype control is shown. Protein expression is the mean fluorescence intensity (MFI) relative to the isotype control. Error bars represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .01. (C) Representative examples of flow cytometry analysis used to assess the frequency of primitive CD34+ human hematopoietic cells that express Bcl-2, Bcl-xL, and Mcl-1. Frequencies represent the percentage of cells positive for the respective protein and CD34 expression ± SEM. Plots are representative of at least 3 independent experiments.
Mcl-1 is required for maintaining the primitive lin−CD34+CD38− SRC-enriched phenotype in vitro
A previous study of the mouse hematopoietic system identified Mcl-1 as an important regulator of primitive mouse HPCs.23 To directly assess whether Mcl-1 is required for mouse HSC repopulating function, we used a cre-excision system in conjunction with lineage-depleted (lin−) Mcl-1 floxed (Mcl-1f/f) BM cells (supplemental Figure 2A-B). Loss of Mcl-1 caused a reduction in the viability and frequency of primitive LSK BM cells (supplemental Figure 2C-E), a reduced frequency of hematopoietic progenitors (supplemental Figure 2F), and resulted in a nearly 6-fold reduction in HSC regenerative capacity (supplemental Figure 2G).
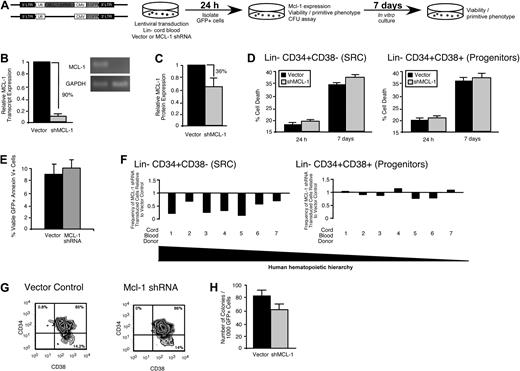
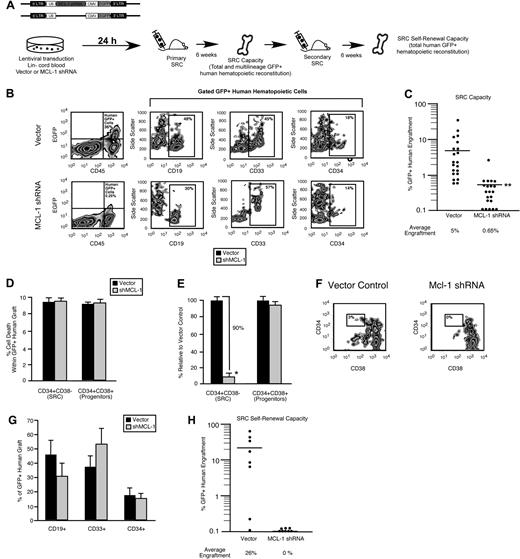
Similar to the mouse LSK compartment, the lin− human hematopoietic compartment is heterogeneous, consisting of both a self-renewing CD34+CD38− multipotent stem cell fraction and a developmentally restricted CD34+CD38+ progenitor fraction.37 To examine the role of Mcl-1 in human HSC and HPC function, we targeted Mcl-1 with a lentiviral vector31 expressing a shRNA (Figure 4A). In contrast to the rapid increase in cell death observed in the mouse HPC fraction less than 24 hours after loss of Mcl-1 expression23 (supplemental Figure 2C) shRNA knockdown of Mcl-1 in the human lin− CD34+ CB cells (Figure 4B-C; supplemental Figure 3) did not affect viability, even after 1 week of ex vivo culture (Figure 4D). Similarly, the percentage of preapoptotic (viable annexin V+) human lin− CD34+ CB cells was not affected by Mcl-1 knockdown (Figure 4E). Although knockdown of Mcl-1 did not affect survival in primitive human hematopoietic cells, it nonetheless caused a rapid reduction in the frequency of lin−CD34+CD38− cells, a population that is highly enriched for human SRCs (Figure 4F left, 7 independent CB donors, G). Comparatively, the frequency of lin−CD34+CD38+ cells, which are enriched for progenitors and largely devoid of SRCs,37 was unaffected by Mcl-1 knockdown (Figure 4F right, G). In agreement with this observation, knockdown of Mcl-1 did not significantly affect the frequency of human hematopoietic progenitors as measured by the colony-forming unit assay12 (Figure 4H). Collectively, these findings indicated that cells found exclusively within the rare the SRC-enriched CD34+CD38− fraction of human CB require Mcl-1 for their in vitro function and that Mcl-1 may have a role is these cells that is unique from regulation of cell survival.
Mcl-1 is required for maintaining the primitive lin− CD34+CD38− SRC-enriched phenotype in vitro. (A) Experimental strategy used to examine the role of Mcl-1 in the viability and function of primitive human HPCs in vitro. (B) Knockdown of the human Mcl-1 transcript in lin− CB hematopoietic cells. Error bars represent the mean ± SEM of 3 independent experiments. (C) Knockdown of human Mcl-1 protein expression in lin− CB hematopoietic cells. Protein expression is the mean fluorescence intensity (MFI) relative to the isotype control. Error bars represent the mean ± SEM of 3 independent experiments. (D) Analysis of the effect of Mcl-1 knockdown on the viability of human lin−CD34+CD38− and CD34+CD38+ CB hematopoietic cells at 24 hours after transduction and after 1 week of ex vivo culture after transduction. Error bars represent the mean ± SEM of 3 independent experiments. (E) Average frequency of viable (7AAD−) annexin V+ human lin− hematopoietic cells 24 hours after transduction with the empty vector or Mcl-1 shRNA-expressing vector. Error bars represent SEM (n = 3). (F) Analysis of the effect of Mcl-1 knockdown on primitive cell phenotypes within the human HSC hierarchy. Analysis was performed on transduced lin− cells isolated from 7 independent CB donors. (G) Representative examples of flow cytometric analyses used to assess the effects of Mcl-1 knockdown on the primitive human hematopoietic phenotypes. Percentages represent frequency of total human cells with indicated cell surface phenotype. (H) Analysis of the effect of Mcl-1 knockdown on the frequency of human hematopoietic progenitors. Progenitor frequency is expressed as the number of hematopoietic colonies scored after 12 to 14 days per 1000 cells plated. Error bars represent the mean ± SEM of 4 independent experiments.
Mcl-1 is required for maintaining the primitive lin− CD34+CD38− SRC-enriched phenotype in vitro. (A) Experimental strategy used to examine the role of Mcl-1 in the viability and function of primitive human HPCs in vitro. (B) Knockdown of the human Mcl-1 transcript in lin− CB hematopoietic cells. Error bars represent the mean ± SEM of 3 independent experiments. (C) Knockdown of human Mcl-1 protein expression in lin− CB hematopoietic cells. Protein expression is the mean fluorescence intensity (MFI) relative to the isotype control. Error bars represent the mean ± SEM of 3 independent experiments. (D) Analysis of the effect of Mcl-1 knockdown on the viability of human lin−CD34+CD38− and CD34+CD38+ CB hematopoietic cells at 24 hours after transduction and after 1 week of ex vivo culture after transduction. Error bars represent the mean ± SEM of 3 independent experiments. (E) Average frequency of viable (7AAD−) annexin V+ human lin− hematopoietic cells 24 hours after transduction with the empty vector or Mcl-1 shRNA-expressing vector. Error bars represent SEM (n = 3). (F) Analysis of the effect of Mcl-1 knockdown on primitive cell phenotypes within the human HSC hierarchy. Analysis was performed on transduced lin− cells isolated from 7 independent CB donors. (G) Representative examples of flow cytometric analyses used to assess the effects of Mcl-1 knockdown on the primitive human hematopoietic phenotypes. Percentages represent frequency of total human cells with indicated cell surface phenotype. (H) Analysis of the effect of Mcl-1 knockdown on the frequency of human hematopoietic progenitors. Progenitor frequency is expressed as the number of hematopoietic colonies scored after 12 to 14 days per 1000 cells plated. Error bars represent the mean ± SEM of 4 independent experiments.
A functional dependence on Mcl-1 for self-renewal capacity defines the human stem cell hierarchy
To examine whether the rapid loss of lin−CD34+CD38− cells after Mcl-1 knockdown translated into a reduction in human SRC regenerative capacity, we implemented our Mcl-1 loss-of-function strategy in human CB SRCs (Figure 5A). In agreement with our phenotypic observations in the SRC-enriched lin−CD34+CD38− fraction (Figure 4F), knockdown of Mcl-1 led to an 8-fold reduction in the hematopoietic regenerative capacity of human SRCs, showing that Mcl-1 is a critical regulator of human SRC regenerative function in vivo (42 mice that received a transplant with the use of 7 independent CB donors; Figure 5B-C). To verify the specificity of our knockdown, we targeted Mcl-1 with a second independent shRNA sequence and observed a similar reduction in human SRC regenerative capacity (supplemental Figure 4). Consistent with our in vitro findings (Figure 4D), knockdown of Mcl-1 did not affect viability within the human CD34+CD38− SRC fraction or human CD34+CD38+ progenitor fraction in vivo (Figure 5D). However, again parallel to our in vitro findings (Figure 4F), knockdown of Mcl-1 caused a 10-fold reduction in the frequency of human cells with the CD34+CD38− SRC-enriched phenotype within the human graft, indicating that Mcl-1 is required for maintaining the most primitive phenotype of the human hematopoietic hierarchy in vivo (Figure 5E-F). In addition, Mcl-1 knockdown did not affect the frequency of lymphoid, myeloid, or total primitive CD34+ cells within the human graft (Figure 5G), indicating that knockdown of Mcl-1 did not affect the ability of primitive human HPCs to differentiate in vivo. Self-renewal is rigorously assessed in human SRCs by their ability to reinitiate hematopoietic grafts after serial passage into secondary NOD/SCID recipients. To directly assay whether the loss of the CD34+CD38− phenotype after Mcl-1 knockdown and the reduced hematopoietic regenerative capacity of Mcl-1–deficient SRCs was due to a decreased capacity for stem cell self-renewal, we performed secondary transplantations as a functional measure of self-renewal. With the use of equal numbers of transplanted cells isolated from primary recipient mice, we found that knockdown of Mcl-1 in human SRCs resulted in the complete ablation of their secondary hematopoietic regenerative capacity, showing that Mcl-1 is essential for the self-renewal capacity of human SRCs (Figure 5H, cells isolated from primary grafts representing 3 independent CB donors) and showing a role for Mcl-1 as a regulator of self-renewal in the human stem cell compartment.
A functional dependence on Mcl-1 for self-renewal capacity hierarchically distinguishes human HSCs from HPCs. (A) Experimental strategy used to examine the role of Mcl-1 in human SRC regenerative function and self-renewal in vivo. (B) Representative examples of flow cytometry analyses used to examine the effect of Mcl-1 knockdown on human SRC regenerative capacity in vivo. Percentages represent frequency of human engrafted cells with indicated cell surface phenotype. (C) Effect of Mcl-1 knockdown on human SRC regenerative capacity in vivo. Each dot represents the frequency of CD45+GFP+ human hematopoietic cells in the BM of 1 primary recipient mouse that received a transplant with Mcl-1 shRNA or vector control transduced SRCs. The frequencies represent the average frequency of GFP+ human hematopoietic cells in the BM of recipient mice. Bars represent the mean of 7 independent experiments, each with 3 mice per group. **P < .01. (D) Analysis of the effect of Mcl-1 knockdown on the viability of engrafted human hematopoietic cells. The average frequency of dead cells in the CD34+CD38− or CD34+CD38+ fraction of the transduced human hematopoietic graft (GFP+7AAD+) in the BM of primary recipient mice that received a transplant with Mcl-1 shRNA or vector control transduced SRCs. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (E) Analysis of the effect of Mcl-1 knockdown on the CD34+CD38− stem cell and CD34+CD38+ progenitor compartments in vivo. The average frequency of CD34+CD38− and CD34+CD38+ cells within the GFP+ human hematopoietic graft in the BM of primary recipient mice reconstituted with Mcl-1 shRNA transduced SRCs is expressed relative to vector control. Error bars represent the mean ± SEM *P < .05. (F) Representative examples of flow cytometric analyses used to examine the effect of Mcl-1 knockdown on the human CD34+CD38− SRC–enriched fraction of the human hematopoietic graft in vivo. Percentages represent frequency of 34+38− cells. (G) Analysis of the effect of Mcl-1 knockdown on multilineage and primitive human hematopoietic engraftment. The average frequency of lymphoid (CD19+), myeloid (CD33+), and primitive (CD34+) cells within the GFP+ human hematopoietic graft (gated CD45+GFP+ cells) in the BM of primary recipient mice reconstituted with vector or Mcl-1 shRNA-transduced SRCs. Error bars represent the mean ± SEM of at least 3 independent experiments. (H) Effect of Mcl-1 knockdown on human SRC self-renewal capacity in vivo. Each dot represents the frequency of CD45+GFP+ human hematopoietic cells in the BM of 1 secondary recipient mouse that received a transplant with human-engrafted BM isolated from a primary recipient mouse that received a transplant with Mcl-1 shRNA or vector control transduced SRCs. The frequencies represent the average frequency of GFP+ human hematopoietic cells in the BM of secondary recipient mice. Equal numbers of GFP+CD45+CD34+ cells isolated from primary grafts representing 3 independent CB donors were used.
A functional dependence on Mcl-1 for self-renewal capacity hierarchically distinguishes human HSCs from HPCs. (A) Experimental strategy used to examine the role of Mcl-1 in human SRC regenerative function and self-renewal in vivo. (B) Representative examples of flow cytometry analyses used to examine the effect of Mcl-1 knockdown on human SRC regenerative capacity in vivo. Percentages represent frequency of human engrafted cells with indicated cell surface phenotype. (C) Effect of Mcl-1 knockdown on human SRC regenerative capacity in vivo. Each dot represents the frequency of CD45+GFP+ human hematopoietic cells in the BM of 1 primary recipient mouse that received a transplant with Mcl-1 shRNA or vector control transduced SRCs. The frequencies represent the average frequency of GFP+ human hematopoietic cells in the BM of recipient mice. Bars represent the mean of 7 independent experiments, each with 3 mice per group. **P < .01. (D) Analysis of the effect of Mcl-1 knockdown on the viability of engrafted human hematopoietic cells. The average frequency of dead cells in the CD34+CD38− or CD34+CD38+ fraction of the transduced human hematopoietic graft (GFP+7AAD+) in the BM of primary recipient mice that received a transplant with Mcl-1 shRNA or vector control transduced SRCs. Error bars represent the mean ± SEM of 3 independent experiments, each with 3 mice per group. (E) Analysis of the effect of Mcl-1 knockdown on the CD34+CD38− stem cell and CD34+CD38+ progenitor compartments in vivo. The average frequency of CD34+CD38− and CD34+CD38+ cells within the GFP+ human hematopoietic graft in the BM of primary recipient mice reconstituted with Mcl-1 shRNA transduced SRCs is expressed relative to vector control. Error bars represent the mean ± SEM *P < .05. (F) Representative examples of flow cytometric analyses used to examine the effect of Mcl-1 knockdown on the human CD34+CD38− SRC–enriched fraction of the human hematopoietic graft in vivo. Percentages represent frequency of 34+38− cells. (G) Analysis of the effect of Mcl-1 knockdown on multilineage and primitive human hematopoietic engraftment. The average frequency of lymphoid (CD19+), myeloid (CD33+), and primitive (CD34+) cells within the GFP+ human hematopoietic graft (gated CD45+GFP+ cells) in the BM of primary recipient mice reconstituted with vector or Mcl-1 shRNA-transduced SRCs. Error bars represent the mean ± SEM of at least 3 independent experiments. (H) Effect of Mcl-1 knockdown on human SRC self-renewal capacity in vivo. Each dot represents the frequency of CD45+GFP+ human hematopoietic cells in the BM of 1 secondary recipient mouse that received a transplant with human-engrafted BM isolated from a primary recipient mouse that received a transplant with Mcl-1 shRNA or vector control transduced SRCs. The frequencies represent the average frequency of GFP+ human hematopoietic cells in the BM of secondary recipient mice. Equal numbers of GFP+CD45+CD34+ cells isolated from primary grafts representing 3 independent CB donors were used.
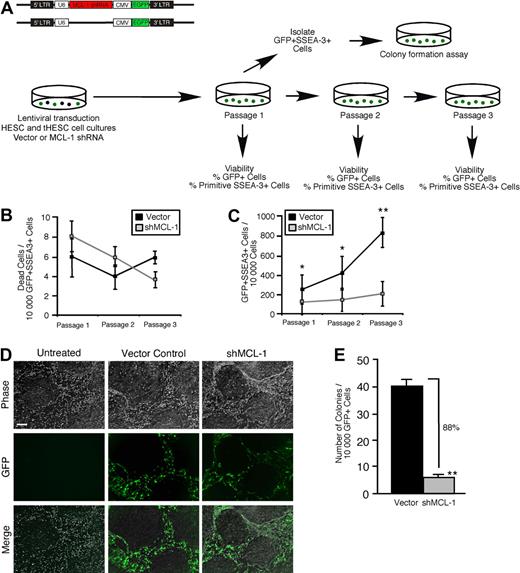
To assess whether the role of Mcl-1 as a regulator of self-renewal function was ontogenetically conserved throughout the human stem cell hierarchy, we implemented our loss-of-function strategy in purified hPSCs in conjunction with established in vitro assays for hPSC function4 (Figure 6A). Although knockdown of Mcl-1 (data not shown) did not affect viability in the primitive self-renewing SSEA-3+ clonogenic fraction of hPSC cultures over 3 passages (Figure 6B), it almost completely abrogated stem cell self-renewal (Figure 6C-D; supplemental Figure 5), indicating that the stem cell fraction in hPSC cultures is highly dependent on Mcl-1 for self-renewal capacity. The ability to reinitiate colonies and reestablish hPSC cultures provides the most rigorous functional measurement of hPSC self-renewal. With the use of the established hPSC colony-initiating cell assay,4 we found that knockdown of Mcl-1 had an extremely potent effect on hPSC self-renewal capacity, resulting in near total loss of the ability of sorted GFP+SSEA-3+ hPSCs to reinitiate colonies after the first passage (Figure 6A,E). Together, these observations established Mcl-1 expression as an absolute requirement for self-renewal in the ontogenetically primitive hPSC fraction and showed a mechanistic conservation of Mcl-1 function in the human stem cell compartment.
Knockdown of Mcl-1 in hPSCs shows a mechanistic conservation of Mcl-1 function as a regulator of self-renewal in human stem cells. (A) Experimental strategy used to examine whether Mcl-1 is required for survival and self-renewal in hPSC cultures. (B) Analysis of the effect of Mcl-1 knockdown on cell viability in the primitive self-renewing SSEA-3+ population of hPSC cultures. hPSC cultures were transduced with the empty vector or Mcl-1 shRNA-expressing vector and analyzed for the frequency of dead cells in the GFP+SSEA-3+ population at each passage. Error bars represent the mean ± SEM of 3 independent experiments. (C) Analysis of the effect of Mcl-1 knockdown on clonogenic self-renewal capacity in the primitive fraction of hPSC cultures. hPSC cultures were transduced with the empty vector or Mcl-1 shRNA-expressing vector and analyzed for the GFP expression in the viable primitive (SSEA-3+) fraction at each passage. The primitive clonogenic fraction is expressed as the frequency of GFP+SSEA-3+ cells per 10 000 cells. Error bars represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .01. (D) Representative examples of hPSC cultures transduced with the empty vector, Mcl-1 shRNA-expressing vector, or untreated. Scale bar represents 50μM. (E) Effect of Mcl-1 knockdown on the frequency of hPSC colony-initiating cells. hPSC cultures were transduced with the empty vector or Mcl-1 shRNA-expressing vector, and the GFP+ SSEA-3+ fraction was analyzed for the frequency of hPSC colony initiating cells after the first passage. Error bars represent the mean ± SEM of 3 independent experiments. **P < .01.
Knockdown of Mcl-1 in hPSCs shows a mechanistic conservation of Mcl-1 function as a regulator of self-renewal in human stem cells. (A) Experimental strategy used to examine whether Mcl-1 is required for survival and self-renewal in hPSC cultures. (B) Analysis of the effect of Mcl-1 knockdown on cell viability in the primitive self-renewing SSEA-3+ population of hPSC cultures. hPSC cultures were transduced with the empty vector or Mcl-1 shRNA-expressing vector and analyzed for the frequency of dead cells in the GFP+SSEA-3+ population at each passage. Error bars represent the mean ± SEM of 3 independent experiments. (C) Analysis of the effect of Mcl-1 knockdown on clonogenic self-renewal capacity in the primitive fraction of hPSC cultures. hPSC cultures were transduced with the empty vector or Mcl-1 shRNA-expressing vector and analyzed for the GFP expression in the viable primitive (SSEA-3+) fraction at each passage. The primitive clonogenic fraction is expressed as the frequency of GFP+SSEA-3+ cells per 10 000 cells. Error bars represent the mean ± SEM of 3 independent experiments. *P < .05, **P < .01. (D) Representative examples of hPSC cultures transduced with the empty vector, Mcl-1 shRNA-expressing vector, or untreated. Scale bar represents 50μM. (E) Effect of Mcl-1 knockdown on the frequency of hPSC colony-initiating cells. hPSC cultures were transduced with the empty vector or Mcl-1 shRNA-expressing vector, and the GFP+ SSEA-3+ fraction was analyzed for the frequency of hPSC colony initiating cells after the first passage. Error bars represent the mean ± SEM of 3 independent experiments. **P < .01.
Discussion
The molecular regulators that control the complex processes of survival, self-renewal, and differentiation that define the human stem cell compartment remain largely unknown. Our study shows a hierarchical dependence of human stem cells on the Bcl-2 family member Mcl-1 for their self-renewal capacity and shows that Mcl-1 is a defining molecular regulator of human stem cell function. Further investigation into the mechanistic regulation of stem cell self-renewal by Mcl-1 as well as the individual roles of other Bcl-2 family members will probably provide additional insights into how survival, self-renewal, and differentiation are regulated in human stem cells. Specifically, our findings with the Bcl-2 family inhibitor obatoclax (Figures 1–2) in combination with our Bcl-2 family expression data (Figure 3) suggest that the other Bcl-2 family members may have important roles in regulating human stem cell function. Particularly, note that expression of Bcl-2 was also highly up-regulated in the CD34+CD38− SRC-enriched and CD34+CD38+ progenitor fractions on in vivo regeneration of human hematopoiesis (Figure 3).
One of the central challenges to studying survival in human stem cells is that they are not defined phenotypically but rather are defined with the use of functional assays such as the NOD/SCID xenotransplantation assay. Therefore, if a stem cell has undergone apoptosis, it is not possible to directly detect this retrospectively per se, but rather it can only be characterized as a function of the available end point readouts in the assay. Although we did not observe any change in the viability of Mcl-1–targeted primitive lin− CB cells in vitro or of human grafts in which Mcl-1 was targeted in vivo (Figures 1,4–5), it is possible that extremely rare cells within the CD34+CD38− fraction may have undergone apoptosis in vivo after loss of Mcl-1. Mechanistically, this decreased survival may have translated into a decreased SRC regenerative function and self-renewal capacity. We were not able to achieve levels of Mcl-1 protein knockdown in primary human lin− CD34+ cells beyond 36%, despite testing 3 shRNA sequences against Mcl-1 (Figure 5C; supplemental Figure 3). Because there are few studies examining gene knockdown in primary human stem cells, it is difficult to characterize the relative efficacy of this knockdown. It may be possible that achieving higher levels of Mcl-1 knockdown could translate into a survival versus a self-renewal effect in the primitive SRC fraction. However, our observation that the human stem cell compartment requires Mcl-1 for self-renewal was not limited to the hematopoietic system, as shown by the potent effect on self-renewal in hPSCs after loss of Mcl-1 function (Figure 6). These analogous findings in more than one type of human stem cell support a conservation of Mcl-1 function as a regulator of self-renewal in the human stem cell compartment. Interestingly, our finding that Mcl-1 has a functional role beyond regulation of cell survival is not without precedent, because a previous study that used Mcl-1 null mice showed that loss of Mcl-1 function resulted in developmental arrest at the blastocyst stage but did not lead to an increase in cell death.41 We found that primitive hPSCs, which are derived from human blastocysts, absolutely required Mcl-1 for self-renewal versus survival (Figure 6). This raises the possibility that Mcl-1 may have a broader, conserved regulatory role in early mammalian ontogeny apart from regulating cell survival.
From a clinical perspective, our findings suggest that therapeutic interventions targeting Mcl-1 or its upstream regulators such as Mcl-1 ubiquitin ligase E342 can be developed to improve the efficiency of HSC transplantations by increasing HSC numbers through increased self-renewal capacity. Intriguingly, Mcl-1 has been shown to be targeted for proteasomal degradation by glycogen synthase kinase 3 (GSK-3) phosphorylation in several human cell lines.43 We have previously established that GSK-3 is another indispensable regulator of primary human stem cell function,12 and inhibition of GSK-3 has shown positive results in augmenting the efficiency of human HSC transplantations in recent clinical trials.44 Further work could be conducted to assess whether GSK-3–dependent regulation of HSC function may involve control of Mcl-1 stability. In addition, Mcl-1 has specifically been implicated as a regulator of survival and transformation in scores of human blood cancers and solid tumors.45 Human malignancies such as AML have been shown to be initiated in a hierarchical manner by rare LSCs analogous to normal HSCs.46 We found that the LSC hierarchy is also defined by a functional dependence on 1 or more Bcl-2 family members, for the first time providing direct experimental evidence that the Bcl-2 regulatory pathway is of key importance in the survival or self-renewal of primary human CSCs. Further work into the roles of individual Bcl-2 family proteins, including Mcl-1, in primary CSCs may therefore be an important factor in designing treatment strategies that specifically target CSCs while leaving other cell populations intact.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gordon Shore of Gemin X Pharmaceuticals for providing obatoclax, Tamra Werbowetski-Ogilvie for valuable comments and feedback on the manuscript, and Andreas Hofmann for providing our laboratory with pLV-CIG.
This work was supported by a research grant from a Canada Research Chair in Stem Cell Biology and Regenerative Medicine, Canadian Institutes of Health Research, and the Ontario Institute for Cancer Research (M.B.) and by graduate research scholarships from Ontario Graduate Scholarship and National Science and Engineering Research Council (C.J.V.C.).
Authorship
Contribution: C.J.V.C. designed experiments, performed research, analyzed and interpreted data, and co-wrote the manuscript; J.B.L. designed experiments, performed research, and analyzed and interpreted data; M.L.-M. and T.W. performed research; A.X. and B.L. provided vital research reagents; and M.B. designed experiments, interpreted data, and co-wrote manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mickie Bhatia, SCC-RI, Faculty of Health Sciences, McMaster University, 1200 Main St W, MDCL 5029, Hamilton, ON, Canada, L8N 3Z5; e-mail: mbhatia@mcmaster.ca.