Abstract
Cutaneous T-cell lymphoma (CTCL) encompasses leukemic variants (L-CTCL) such as Sézary syndrome (SS) and primarily cutaneous variants such as mycosis fungoides (MF). To clarify the relationship between these clinically disparate presentations, we studied the phenotype of T cells from L-CTCL and MF. Clonal malignant T cells from the blood of L-CTCL patients universally coexpressed the lymph node homing molecules CCR7 and L-selectin as well as the differentiation marker CD27, a phenotype consistent with central memory T cells. CCR4 was also universally expressed at high levels, and there was variable expression of other skin addressins (CCR6, CCR10, and CLA). In contrast, T cells isolated from MF skin lesions lacked CCR7/L-selectin and CD27 but strongly expressed CCR4 and CLA, a phenotype suggestive of skin resident effector memory T cells. Our results suggest that SS is a malignancy of central memory T cells and MF is a malignancy of skin resident effector memory T cells.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of non-Hodgkin lymphomas thought to represent malignancies of skin homing T cells.1 CTCL encompasses such diverse presentations as Sézary syndrome (SS/L-CTCL) in which patients present with erythroderma, lymphadenopathy, and circulating clonal malignant T cells, and mycosis fungoides (MF), a variant in which malignant cells reside primarily in infiltrated skin lesions. Although early-stage MF and L-CTCL have previously been considered to be points in a disease continuum, differing molecular profiles and responses to therapy have provided new evidence that MF and L-CTCL may be distinct diseases.2–4 We present here evidence that the malignant T cells in L-CTCL express markers of central memory T cells and those in MF express markers of skin resident effector memory T cells.
Methods
The protocols of this study were performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Partners Human Research Committee (Partners Research Management, Boston, MA). Normal human skin was obtained from patients undergoing cosmetic surgery procedures. Blood and lesional skin were obtained from patients seen at the Dana-Farber/Brigham and Women's Cancer Center Cutaneous Lymphoma Program. L-CTCL and MF patients described in this manuscript met the World Health Organization–European Organization for Research and Treatment of Cancer criteria for L-CTCL/SS or MF.5 Peripheral blood mononuclear cells were isolated by Ficoll centrifugation, and T cells were isolated from skin using short-term explant cultures (1-3 weeks) as described.6 T-cell receptor (TCR) Vβ expression was determined by staining with Vβ specific monoclonal antibody (Beckman Coulter). CCR10 monoclonal antibody (clone 1B57 ) was obtained from Millennium Pharmaceuticals; all other antibodies were obtained from BD Biosciences, eBioscience, or R&D Systems. Isotype-matched negative control antibodies were used to set the gates for positive staining.
In vitro chemotaxis assays were performed as described7 using recombinant CCL22 and CCL21 (R&D Systems). Gene expression analysis of CD27 was performed using highly purified flow sorted T cells: CD4+ T cells from MF skin lesions (5 donors), normal skin (3 donors), and clonal malignant T cells from blood in L-CTCL patients (2 donors). Whole genome Agilent microarray analysis was performed (Miltenyi Biotec).
Results and discussion
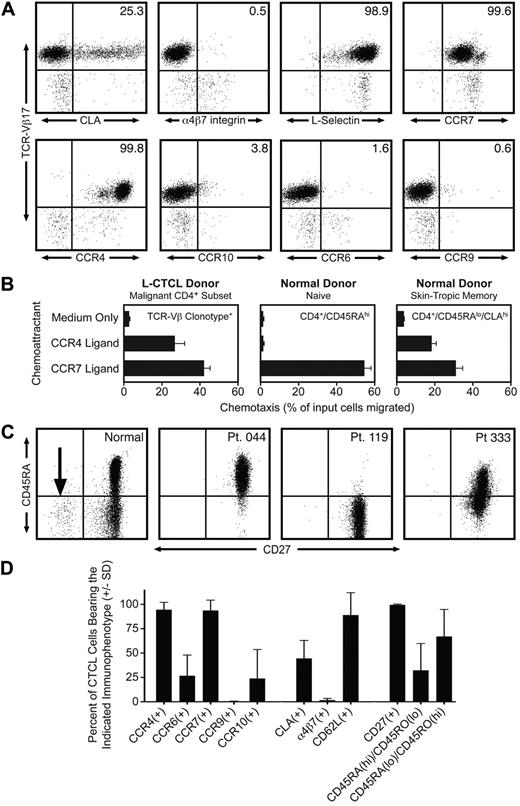
By staining with a panel of monoclonal antibodies directed against specific TCR-Vβ subfamilies, we identified 12 patients with known L-CTCL who had a clonal CD4 T-cell subset composing more than 50% of the total T-cell population. Clonal malignant T cells in these patients expressed uniformly high levels of CCR4, but variable to low levels of other skin homing addressins, including CLA, CCR10, and CCR6 (Figure 1A,D). There was no expression of the gut homing addressins α4β7-integrin and CCR9, consistent with a lack of mucosal tropism. One striking feature of the malignant clones was the universal coexpression of the lymph node homing receptors CCR7 and L-selectin (Figure 1A,D). Twelve of 12 malignant clones expressed both CCR7 and L-selectin. L-selectin and CCR7 are both considered essential for T-cell homing to lymph nodes from blood through high endothelial venules.8 L-selectin and CCR7 are coexpressed by both naive T cells and a subset of memory T cells termed central memory T (Tcm) cells.8 A subset of Tcm cells in blood also coexpress CLA and CCR4 and thus should be able to migrate into both the skin and lymph nodes.9 Approximately 20% of the T cells resident in normal human skin have this TCM phenotype.10 The differentiation marker CD27 is also expressed at high levels by Tcm cells and is lost on differentiation into effector memory T cells.11 We observed universal and high CD27 expression in the clonal malignant T cells from 12 of 12 L-CTCL patients (Figure 1C-D), consistent with a TCM phenotype. In contrast, there was significant heterogeneity in CD45RO and CD45RA expression. Malignant T cells from L-CTCL patients expressed the naive T-cell marker CD45RA, the memory T-cell marker CD45RO, or combinations of the 2 (Figure 1C-D). Lastly, we established the functionality of CCR4 and CCR7 expressed universally by malignant L-CTCL clones by demonstrating their ability to support chemotaxis in vitro (Figure 1B). L-CTCL clones migrated even more efficiently to CCR4 ligand than normal skin-tropic (CLA+) T cells from blood and also displayed significant migration to CCR7 ligand.
Malignant T cells from the blood of patients with L-CTCL have a central memory T-cell phenotype. (A) Flow cytometric analysis of clonal malignant T cells isolated from the blood of an L-CTCL patient with a TCR-Vβ17 malignant clone demonstrated that malignant T cells expressed high levels of the central memory T-cell markers L-selectin and CCR7. CCR4 was also expressed at high levels, but expression of other skin homing addressins (CLA, CCR6, and CCR10) was variable. There was no detectable expression of the gut homing addressins α4β7-integrin and CCR9. (B) CCR4 and CCR7 expressed by malignant T cells in L-CTCL were functional as demonstrated by their ability to support migration to the CCR4 ligand CCL22 and the CCR7 ligand CCL21 in in vitro T-cell migration assays. Shown are the mean ± SD migration of clonal malignant T cells from 3 L-CTCL donors (left panel) and the naive (center panel) and CLA+ memory T cells (right panel) from 3 normal donors. As expected, normal naive T cells responded only to CCR7 ligand. (C) Clonal malignant T cells from L-CTCL patients expressed high levels of CD27, consistent with a central memory T-cell phenotype, but expression of CD45RA varied among patients. CD4+ CD3+ T cells from a normal donor are shown on the left. The CD27-negative effector T-cell population observed in normal donors is indicated by an arrow. The right 3 panels show 3 L-CTCL patients, and histograms are gated to show only the CD4+ clonal malignant T-cell populations. CD27 was uniformly expressed on malignant cells from all donors, but the expression of CD45RA was variable. (D) Analysis of 11 additional L-CTCL patients with identifiable malignant clones produced similar results. Shown are the mean ± SD of surface marker expression of the CD3+/CD4+ cells expressing the expanded TCR-Vβ clonotype.
Malignant T cells from the blood of patients with L-CTCL have a central memory T-cell phenotype. (A) Flow cytometric analysis of clonal malignant T cells isolated from the blood of an L-CTCL patient with a TCR-Vβ17 malignant clone demonstrated that malignant T cells expressed high levels of the central memory T-cell markers L-selectin and CCR7. CCR4 was also expressed at high levels, but expression of other skin homing addressins (CLA, CCR6, and CCR10) was variable. There was no detectable expression of the gut homing addressins α4β7-integrin and CCR9. (B) CCR4 and CCR7 expressed by malignant T cells in L-CTCL were functional as demonstrated by their ability to support migration to the CCR4 ligand CCL22 and the CCR7 ligand CCL21 in in vitro T-cell migration assays. Shown are the mean ± SD migration of clonal malignant T cells from 3 L-CTCL donors (left panel) and the naive (center panel) and CLA+ memory T cells (right panel) from 3 normal donors. As expected, normal naive T cells responded only to CCR7 ligand. (C) Clonal malignant T cells from L-CTCL patients expressed high levels of CD27, consistent with a central memory T-cell phenotype, but expression of CD45RA varied among patients. CD4+ CD3+ T cells from a normal donor are shown on the left. The CD27-negative effector T-cell population observed in normal donors is indicated by an arrow. The right 3 panels show 3 L-CTCL patients, and histograms are gated to show only the CD4+ clonal malignant T-cell populations. CD27 was uniformly expressed on malignant cells from all donors, but the expression of CD45RA was variable. (D) Analysis of 11 additional L-CTCL patients with identifiable malignant clones produced similar results. Shown are the mean ± SD of surface marker expression of the CD3+/CD4+ cells expressing the expanded TCR-Vβ clonotype.
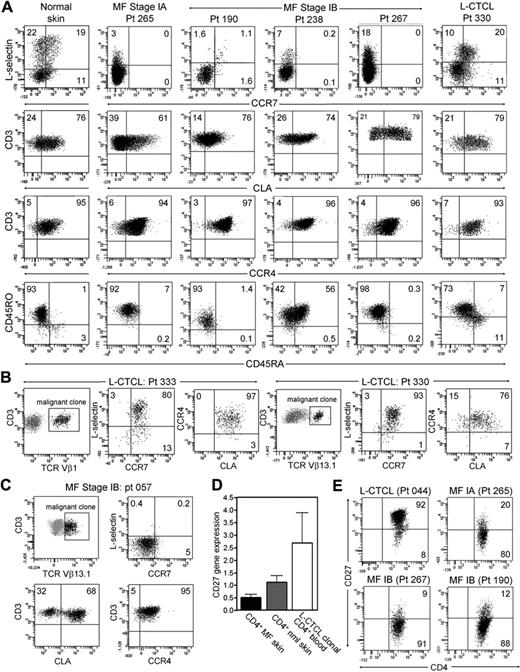
We next isolated and characterized the T cells from normal skin, from the skin lesions of patients with stable MF, a disease stage in which malignant T cells reside in fixed patches or plaques on the skin, and from the skin lesions of patients with L-CTCL. Both normal human skin and the skin lesions of patients with L-CTCL contained clear populations of L-selectin/CCR7–coexpressing Tcm cells (Figure 1A). In marked contrast, T cells isolated from the skin lesions of MF patients had no discernible Tcm cells. T cells shown in Figure 2 are gated to display only CD4+ T cells; this would include both malignant clonal CD4 cells and benign reactive CD4 T cells. A malignant T-cell clone was not identifiable by staining for TCR-Vβ subfamilies in the majority of early-stage MF patients, preventing selective analysis of the malignant clone. However, it was clear from the near-complete absence of L-selectin/CCR7 coexpression that Tcm cells were not frequent in either population. In contrast, T cells from normal skin, MF skin lesions, and the skin lesions of patients with L-CTCL all expressed high levels of the skin homing addressins CLA and CCR4. As observed in L-CTCL patients, expression of CD45RO and CD45RA varied among patients.
CD4+ T cells from MF skin lesions have an effector memory phenotype, but T cells from L-CTCL skin lesions have central memory characteristics. (A) Normal skin and the skin lesions of patients with L-CTCL contained a clear population of L-selectin and CCR7 coexpressing central memory T cells. However, T cells isolated from the skin lesions of stable patch plaque MF lacked coexpression of the central memory markers CCR7/L-selectin but did express the skin homing addressins CCR4 and CLA. All histograms are gated to show only CD4+ T cells. CCR4 was expressed by virtually all T cells, whereas CLA expression was frequent but not universal. Similar to results in L-CTCL patients, expression of CD45RO and CD45RA varied among patients. Four representative patients are shown; similar results were obtained in a total of 15 patients. (B) Clonal malignant T cells in the skin lesions of patients with L-CTCL expressed both L-selectin/CCR7 and skin homing addressins. Malignant T cells from the skin lesions of patients with L-CTCL were selectively studied by gating on T cells expressing the malignant Vβ subfamily (black cells, Vβ1 for patient 333 and Vβ13.1 for patient 330). Histograms are gated to show only malignant T cells. Malignant clonal T cells showed near-universal expression of the central memory markers L-selectin and CCR7 as well as high expression of the skin homing addressins CLA and CCR4. Results from 2 patients are shown; similar findings were observed in T cells isolated from the skin lesions of 4 additional L-CTCL patients. (C) In contrast, clonal T cells arising in MF skin lesions lacked central memory markers. Clonal malignant T cells expressing TCR-Vβ13.1 were evident in the skin lesions of a patient with MF (black cells represent remaining histograms gated to display only clonal malignant cells). Clonal malignant T cells lacked expression of the central memory markers L-selectin/CCR7 but expressed CCR4, and the majority coexpressed CLA. (D) Microarray gene expression analysis demonstrated that T cells from MF skin lesions expressed low levels of CD27 compared with clonal malignant T cells isolated from the blood of L-CTCL patients. The mean ± SEM of 5 MF patients, 3 normal skin patients, and 3 L-CTCL patients are shown. (E) Flow cytometric staining of T cells from the skin lesions of MF and L-CTCL confirmed that CD4+ T cells in MF show loss of CD27, consistent with an effector memory phenotype. T cells from the skin lesion of a representative patient with L-CTCL expressed CD27.
CD4+ T cells from MF skin lesions have an effector memory phenotype, but T cells from L-CTCL skin lesions have central memory characteristics. (A) Normal skin and the skin lesions of patients with L-CTCL contained a clear population of L-selectin and CCR7 coexpressing central memory T cells. However, T cells isolated from the skin lesions of stable patch plaque MF lacked coexpression of the central memory markers CCR7/L-selectin but did express the skin homing addressins CCR4 and CLA. All histograms are gated to show only CD4+ T cells. CCR4 was expressed by virtually all T cells, whereas CLA expression was frequent but not universal. Similar to results in L-CTCL patients, expression of CD45RO and CD45RA varied among patients. Four representative patients are shown; similar results were obtained in a total of 15 patients. (B) Clonal malignant T cells in the skin lesions of patients with L-CTCL expressed both L-selectin/CCR7 and skin homing addressins. Malignant T cells from the skin lesions of patients with L-CTCL were selectively studied by gating on T cells expressing the malignant Vβ subfamily (black cells, Vβ1 for patient 333 and Vβ13.1 for patient 330). Histograms are gated to show only malignant T cells. Malignant clonal T cells showed near-universal expression of the central memory markers L-selectin and CCR7 as well as high expression of the skin homing addressins CLA and CCR4. Results from 2 patients are shown; similar findings were observed in T cells isolated from the skin lesions of 4 additional L-CTCL patients. (C) In contrast, clonal T cells arising in MF skin lesions lacked central memory markers. Clonal malignant T cells expressing TCR-Vβ13.1 were evident in the skin lesions of a patient with MF (black cells represent remaining histograms gated to display only clonal malignant cells). Clonal malignant T cells lacked expression of the central memory markers L-selectin/CCR7 but expressed CCR4, and the majority coexpressed CLA. (D) Microarray gene expression analysis demonstrated that T cells from MF skin lesions expressed low levels of CD27 compared with clonal malignant T cells isolated from the blood of L-CTCL patients. The mean ± SEM of 5 MF patients, 3 normal skin patients, and 3 L-CTCL patients are shown. (E) Flow cytometric staining of T cells from the skin lesions of MF and L-CTCL confirmed that CD4+ T cells in MF show loss of CD27, consistent with an effector memory phenotype. T cells from the skin lesion of a representative patient with L-CTCL expressed CD27.
We then selectively studied the phenotype of clonal malignant T cells from L-CTCL and MF skin lesions. Clonal malignant T cells were studied from 6 L-CTCL patients with known malignant clones; 2 representative donors are shown (Figure 2B). Clonal malignant T cells from the skin lesions of these patients expressed almost universally high levels of L-selectin and CCR7, a phenotype suggestive of Tcm cells. Lesional T cells also expressed high levels of CLA, in contrast to the more variable expression of CLA observed when clonal cells were isolated from peripheral blood (Figure 1). Thus, the malignant T cells in L-CTCL maintain their TCM phenotype even after entering the skin. However, it appears that only malignant T cells expressing high levels of CLA are capable of migrating into skin.
In occasional patients with MF, a malignant clone was identifiable by flow cytometry. One such patient (patient 057) had lesional malignant T cells identifiable by their expression of TCR-Vβ13.1 (Figure 2C). Selective gating on these clonal T cells demonstrated that they lacked coexpression of L-selectin/CCR7 but that all expressed CCR4 and the majority coexpressed CLA.
Expression of tissue addressins such as CLA and the lack of L-selectin/CCR7 coexpression are characteristics of effector memory T (Tem) cells.8 Tem cells generated by antigen-specific immune responses persist long-term in peripheral tissues, such as the skin, and have recently been shown to remain in a fixed location once they enter peripheral tissues.12–16 Consistent with a phenotype of TEM, the T cells from MF skin lesions also lacked expression of CD27, as demonstrated by gene expression analysis and direct protein demonstration by flow cytometry (Figure 2D-E).
L-CTCL and MF exhibit many critical clinical differences. In L-CTCL, malignant T cells accumulate in the blood and lymph nodes and give rise to diffuse erythema of the skin. L-CTCL is often refractory to multiple therapies, and such patients can require hematopoietic stem cell transplantation.17 The median survival for patients with SS is 3 years, and patients die most commonly from infections associated with immunosuppression.18,19 In MF patients, malignant T cells are restricted to fixed patches and plaques within the skin that can remain stable in size and location for many years. MF often responds favorably to the same skin-directed therapies used to treat other inflammatory skin diseases, including topical steroids and phototherapy. The majority of patients diagnosed with early-stage MF will have a normal life expectancy.3
We present evidence that the differing clinical and biologic behavior of L-CTCL and MF probably reflects the fact that malignant T cells in these disorders arise from 2 distinct T-cell subsets. The cutaneous subset of TCM has a high proliferative potential and actively recirculates between the blood, lymph nodes, and skin.10,20 We find that the malignant T cells in L-CTCL (SS) have a TCM phenotype, consistent with the clinical presentation of peripheral blood disease, lymphadenopathy, and diffuse erythroderma of the skin. In contrast, skin resident Tem cells are polarized effector T cells, a population of T cells that produce inflammatory cytokines and, according to recent murine models, remain stationary within a particular location in the skin.14,16 We find that T cells from MF skin lesions have a TEM phenotype. This is remarkably consistent with their clinical ability to produce inflamed skin lesions and to recruit nonmalignant T cells into the skin, giving rise to inflammatory patches and plaques.21 Because of the sessile, nonmotile nature of the majority of Tem cells, plaques often remain in fixed anatomic locations for many years. Moreover, malignant T cells remain confined to the skin and do not enter the peripheral blood or lymph nodes.
Our findings together with those from other groups suggest that L-CTCL and MF should be considered as separate lymphomas arising from distinct functional T-cell subsets. Unlike other malignancies, CTCL does not necessarily progress from early- to late-stage disease in a stepwise fashion. Many patients with L-CTCL present de novo with erythroderma and peripheral blood involvement.3 The majority of patients diagnosed with early-stage MF will never progress to advanced-stage disease. It stands to reason that any T-cell subset trafficking through the skin is susceptible to malignant transformation. Normal human skin contains both the effector and central memory T cells.10 By acknowledging the distinct cell of origin of these 2 cutaneous T-cell lymphomas, we can gain additional insights into their biology and novel perspectives on therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who donated the skin and blood samples that made this work possible; Dr Jessica Teague, who provided editorial assistance; and Dr Thomas Cochran of the Boston Center for Plastic Surgery and Dr Elof Eriksson of Brigham and Women's Hospital who generously provided adult human skin samples. The authors also wish to thank the following members of the Dana-Farber/Brigham and Women's Cancer Center Cutaneous Lymphoma team: Marianne Tawa, NP, Michelle Walsh, Natalie Adams, David C. Fisher, MD, and Corey S. Cutler, MD.
This work was supported by the National Institutes of Health/National Cancer Institute (SPORE in Skin Cancer P50 CA9368305; T.S.K.), National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01 A1025082; T.S.K.), a Damon Runyon Clinical Investigator Award (R.A.C.), a Translational Research Program Grant from the Leukemia & Lymphoma Society (R.A.C.), National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR056720; R.A.C.) and the National Institutes of Health (R01 AI46784; J.J.C.).
National Institutes of Health
Authorship
Contribution: T.S.K., R.A.C., and J.J.C. conceived the experimental plan, designed the experiments, and reviewed and analyzed the experimental results; J.J.C. performed experiments in Figure 1 and helped revise the manuscript; R.A.C. performed experiments in Figure 2 and drafted and revised the manuscript; R.W. performed experiments that are included in Figure 2; and T.S.K. provided patient samples and experimental advice and edited the manuscript.
Conflict-of-interest disclosure: R.A.C. served previously on the scientific advisory board for Therakos. The remaining authors declare no competing financial interests.
Correspondence: Rachael A. Clark, Department of Dermatology, Brigham and Women's Hospital, 77 Ave Louis Pasteur, Rm 671, Boston, MA 02115; e-mail: rclark1@partners.org; or Thomas S. Kupper, Department of Dermatology, Brigham and Women's Hospital, 77 Ave Louis Pasteur, Rm 671, Boston, MA 02115; e-mail: tkupper@partners.org.
References
Author notes
J.J.C. and R.A.C. contributed equally to this study and should be considered as co–first authors.