Abstract
Many features of T-cell homeostasis in primates are still unclear, thus limiting our understanding of AIDS pathogenesis, in which T-cell homeostasis is lost. Here, we performed experiments of in vivo CD4+ or CD8+ lymphocyte depletion in 2 nonhuman primate species, rhesus macaques (RMs) and sooty mangabeys (SMs). Whereas RMs develop AIDS after infection with simian immunodeficiency virus (SIV), SIV-infected SMs are typically AIDS-resistant. We found that, in both species, most CD4+ or CD8+ T cells in blood and lymph nodes were depleted after treatment with their respective antibodies. These CD4+ and CD8+ lymphocyte depletions were followed by a largely lineage-specific CD4+ and CD8+ T-cell proliferation, involving mainly memory T cells, which correlated with interleukin-7 plasma levels. Interestingly, SMs showed a faster repopulation of naive CD4+ T cells than RMs. In addition, in both species CD8+ T-cell repopulation was faster than that of CD4+ T cells, with CD8+ T cells reconstituting a normal pool within 60 days and CD4+ T cells remaining below baseline levels up to day 180 after depletion. While this study revealed subtle differences in CD4+ T-cell repopulation in an AIDS-sensitive versus an AIDS-resistant species, such differences may have particular relevance in the presence of active SIV repli cation, where CD4+ T-cell destruction is chronic.
Introduction
The size and composition of the pool of mature T lymphocytes are tightly regulated by complex homeostatic mechanisms, with the total number of T cells remains relatively constant overtime in healthy individuals.1–3 The numeric homeostasis of mature T cells is required for a normal immune function, with several human diseases being associated with failure of T-cell homeostasis. In the presence of an acute or chronic depletion of T cells, the overall homeostasis of the T-cell compartment can be maintained essentially in 3 ways: (1) by generation of naive CD4+ or CD8+ T cells from thymic precursors, (2) by an increased longevity of T cells, or (3) by peripheral expansion, that is, proliferation of mature T lymphocytes.4,5 The latter phenomenon is defined as “homeostatic proliferation” or “lymphopenia-induced proliferation,” that is, a spontaneous, antigen-independent proliferation of mature T cells, and appears to be the most rapid component of T-cell immune reconstitution after experimental depletion.4,6
Despite its central role in maintaining immune function, the mechanisms regulating the homeostasis of T cells in vivo are still largely unclear. The majority of the relevant studies were conducted in mice, where lymphocyte depletion is induced experimentally (ie, using antibody-mediated depletion or irradiation) or by genetically engineering mice that are unable to produce mature T cells (ie, RAG−/−). Of note, it is still unclear what molecular and cellular mechanisms trigger the proliferation of mature T cells in the event of depletion. It is well established that cytokines, such as IL-7, IL-15, and others, are crucial for T-cell homeostasis. Indeed, plasma concentration of IL-7 and IL-15 is higher in condition of T-cell depletion, a phenomenon that may be related to decreased consumption of these cytokines or, alternatively, to increased production by cell type(s), not yet defined, that senses T-cell depletion.7–11 However, it is not clear whether the requirements for T-cell homeostasis are different for CD4+ and CD8+ T cells and how the homeostasis of the different CD4+ and CD8+ T-cell subsets (namely naive, effector, and memory cells) is regulated. In addition, it has not been determined to what extent the homeostatic response that follows T-cell depletion is lineage-specific (ie, whether only the depleted cell type reconstitutes the compartment) or blind (ie, whether nondepleted cells may occupy the “available space”). These questions are even more difficult to answer in primates, either human or nonhuman, because transgenic/knock out systems are not available to aid experimental approaches. As a result, our knowledge of the regulation of homeostasis of T cells in primates is still very limited.
Pathogenic HIV infection in humans, as well as SIV infections in non-naturally adapted hosts, such as rhesus macaques (RMs), are characterized by progressive CD4+ T-cell depletion and AIDS (reviewed in Grossman et al12 and Douek et al13 ). An important factor contributing this CD4+ T-cell depletion is an insufficient CD4+ T-cell reconstitution, that is, a substantial failure of the regenerative capacity of the lymphoid system to produce enough T cells to compensate for their loss.14–20 The role of an insufficient CD4+ T-cell reconstitution in AIDS pathogenesis has been recently emphasized by the observation that disease progression is ultimately associated with homeostatic failure at the level of central-memory CD4+ T cells, even though the majority of CD4+ T cells that are either killed by the virus or succumb to apoptosis show an effector or effector-memory phenotype.21 The mechanisms responsible for the failure of T-cell reconstitution during HIV infection are complex, and likely they involve the presence of aberrant chronic immune activation, extensive recruitment of naive and central memory CD4+ and CD8+ T cells into the effector compartment, reduced input of naive T cells from the thymus, and disruption of the bone marrow (BM) function and lymph node (LN) microenvironment.22–25 Failure of CD4+ T-cell homeostasis precedes the development of clinically defined AIDS by approximately 1-2 years, and appears to be a critical step in HIV disease progression.19,20 Important advances in our understanding of the pathogenesis of HIV-infection in humans have been provided by studies of primate species that are natural hosts for SIV, such as sooty mangabeys (SMs).26,27 In marked contrast to HIV/SIV-infected humans or RMs, SIV-infected SMs generally maintain normal CD4+ T-cell counts and do not progress to AIDS (reviewed in Paiardini et al26 ). Extensive characterization of SIV infection of SMs has shown that high levels of virus replication, lack of immune control, short lifespan of infected cells in vivo, tropism for CD4+ T cells, and depletion of mucosal CD4+ T cells are all common features of progressive and nonprogressive HIV/SIV infections.26 Based on these findings, the current paradigm maintains that the continuous replication of a cytopathic virus that targets activated/memory CD4+ T cells is not per se sufficient to cause AIDS during a primate lentiviral infection, and predicates that additional cofactors are critical in HIV pathogenesis. Of note, SIV infection of SMs is typically associated with the preservation of a normal lymphoid regenerative compartment and low levels of immune activation,26,28 which may account, at least in part, for the preservation of T-cell homeostasis in these animals. In addition, we recently showed that, in nonhuman primates, the BM is a preferential site for T-cell proliferation under depleting conditions, and that in naturally SIV-infected SMs a preserved BM function is instrumental in maintaining CD4+ T-cell homeostasis.29
In the current study, we attempted to further elucidate the features of T-cell homeostasis in nonhuman primates, and investigate whether the capacity to maintain T-cell homeostasis differs between SMs and RMs, by conducting experiments of in vivo antibody-mediated depletion of either CD4+ or CD8+ lymphocytes.
Methods
Animals
Six SIV-uninfected SMs and 6 SIV-uninfected RMs housed at the Yerkes Primate Research Center (Atlanta, GA) were included in this study. The SMs and RMs of the CD4+ lymphocyte depletion study were, on average, 5.1 and 4.3 years old, respectively; the SMs and RMs of the CD8+ lymphocyte depletion study were, on average, 4.3 and 3.9 years old. The ages of these SMs were similar to those at which they become naturally infected with SIV. The average lifespan of healthy, SIV-uninfected RMs and SMs is approximately 20 years (J.G.E., written communication, April 2010). All animal studies were approved by the University of Pennsylvania and Emory University Institutional Animal Care and Use Committees.
Lymph node biopsies
Lymph node (LN) biopsies and isolation of LN-derived T cells were conducted as described previously.28
Depletion of CD4+ or CD8+ lymphocytes in vivo
Depletion of CD4+ and CD8+ lymphocyte were performed using 10 mg/Kg intravenous anti-CD4 mAb (OKT4A) on day −10 and 5 mg/Kg on days −7, −3, and 0, while for CD8+ lymphocyte depletion animals were treated with 4 mg/Kg intravenous anti-CD8 mAb (OKT8F) on days −2, −1, and 0, a protocol that has been shown to deplete CD4+ and CD8+ lymphocytes in vivo in both RMs and SMs.30–32 We have previously shown that the depleting antibodies used in this study did not mask staining with the fluorescent-labeled anti-CD4 or anti-CD8 antibodies used to assess the levels of these cells.30,33 Of note, the protocol used for CD4+ lymphocyte depletion does not deplete monocytes30 or dendritic cells (data not shown). Blood and LN collection were performed at baseline and at different time points after the last antibody administration.
Flow cytometry
The immunophenotype of lymphocytes derived from PB and LN was analyzed by 4- and 7-color flow cytometric analysis. The staining was performed according to standard procedures using a panel of monoclonal antibodies that have been shown to be cross-reactive with SMs and RMs.29,30 The antibodies used in the study included: anti-CD3 FITC, PerCP, AlexaFluor-700 and APC (SP34); anti-CD4 PerCP and PerCP-Cy5.5 (L200), and anti-CD4 APC-Cy7 (SK3); anti-CD8 APC and PE (OKT8), and anti-CD8 Pacific Blue (RPA-T8); anti-CD28 FITC and PE-Cy7 (CD28.2); anti-CD95 APC (DX2); anti-CD62L PE (SK11); anti-CCR7 FITC and PE (150503), and anti-CCR7 PE-Cy7 (3D12); anti-Ki67 FITC (B56); anti-CD127 PE (R34.34). Data were acquired on a FACScaliber cytometer driven by the CellQuest software (4 colors), and on a LSRII flow cytometer driven by the DiVA software package (7 colors; Becton Dickinson). Analysis of the acquired data were performed using FlowJo software (TreeStar).
Plasma levels of IL-7 and IL-15
IL-7 and IL-15 quantitation was performed as described in Muthukumar et al.34
Statistical analysis
The analyses performed include the 2-tailed Student t test or the Mann-Whitney test for comparisons between groups. Partial correlations that take into account the longitudinal structure of the study (ie, adjusted for repeated measure of the same animal) were computed between variation of IL-7 and CD4+ T-cell counts and fraction of CD4+Ki-67+ T cells. All analyses were performed using Prism (GraphPad Software Inc) or SAS 9.1 (SAS Institute Inc.) software. A P value less than .05 was considered significant.
Mathematical model
Reconstitution data from individual animals were fitted to a bilinear model, in which a first and second rate of increase of cell numbers was distinguished. The moment at which the model switches from the first to the second slope was determined by the fitting procedure. For the reconstitution curves, all time points at which cell numbers were not yet consistently increasing were ignored. The best fits to the experimental data were determined using least square minimization, using the DNLS1 subroutine.35 Ninety-5 percent confidence intervals for the inferred parameters were then determined using a bootstrap method,36 where the residuals to the optimal fit were resampled 500 times.
Results
Antibody-mediated CD4+ and CD8+ lymphocyte depletion in nonhuman primates
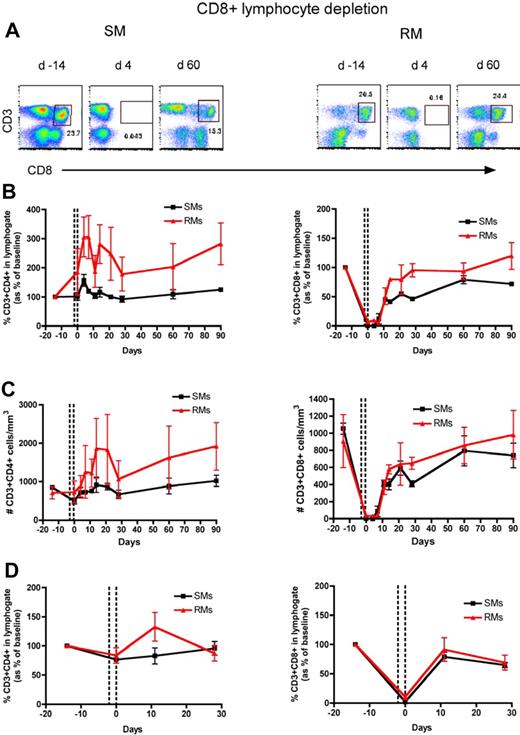
To directly assess the homeostatic regulation of T lymphocytes in nonhuman primates, we used an experimental system in which a transient but severe depletion of CD4+ or CD8+ T cells is induced by administering OKT4A or OKT8F monoclonal antibody, respectively. The OKT4A MAb was administered 4 times in a 10-day period (days −10, −7, −3, 0) in 3 SIV-uninfected RMs and 3 SIV-uninfected SMs, and the OKT8F MAb was administered for 3 consecutive days (days −2, −1, 0) in 3 additional SIV-uninfected RMs and SMs. In all cases, we used flow cytometry to assess CD4+ and CD8+ T-cell depletion and reconstitution in peripheral blood (PB) and lymph nodes (LN), and observed a profound depletion of the targeted T-cell subset (Figures 1A and 2A show representative examples of the levels of CD4+ and CD8+ T cells predepletion and at an early and a later time point after depletion). More specifically, the predepletion percentage of CD3+CD4+ T cells in PB was 20.6% plus or minus 7.9% (mean ± SD) in SMs and 30.8% plus or minus 3.4% in RMs; and in LNs it was 47.9% plus or minus 14.3% in SMs and 41% plus or minus 4.7% in RMs. The percentage of CD3+CD8+ T cells at baseline in PB was 25.3% plus or minus 1.9% in SMs and 17.3% plus or minus 3% in RMs; in LNs it was 19% plus or minus 0.3% in SMs and 17.9% plus or minus 4.6% in RMs. As shown in Figures 1 and 2, the depleting antibodies induced a rapid, lineage-specific (ie, mainly involving CD4+ T cells in the event of CD4+ lymphocyte depletion and CD8+ T cells in the event of CD8+ lymphocyte depletion), and profound depletion of CD4+ or CD8+ T cells. For circulating CD4+ T cells, the nadir value after depletion was 15.1% plus or minus 8.9% of baseline levels in SMs and 3.6% plus or minus 3.3% of baseline levels in RMs (Figure 1B), which corresponded to 56.1 plus or minus 29.1 cells/μL in SMs and 33.3 plus or minus 30.7 cells/μL in RMs (Figure 1C). For LN CD4+ T cells the nadir value was 36.7% plus or minus 6.7% and 33.4% plus or minus 8.6% of baseline in SMs and RMs, respectively (Figure 1D). For circulating CD8+ T cells, the nadir value was 0.1% plus or minus 0.1% of baseline in SMs and 3.8% plus or minus 1.5% of baseline in RMs (Figure 2B), which corresponded to 0.8 plus or minus 0.1 cells/μL in SMs and 18.4 plus or minus 10.3 cells/μL in RMs (Figure 2C). For LN CD8+ T cells the nadir value was 2.1% plus or minus 0.6% and 10.6% plus or minus 4.1% of baseline in SMs and RMs, respectively (Figure 2D). In general, we observed a less efficient depletion in LNs than in blood, a finding that was particularly evident for CD4+ T cells. Of note, none of the experiments depleted the nontargeted populations (ie, CD8+ T cells during anti-CD4 treatment or CD4+ T cells during anti-CD8 treatment). Collectively, these data indicate that both anti-CD4 and anti-CD8 antibodies are effective in depleting T cells in primates, with the loss of CD8+ T cells being more profound than that of CD4+ T cells, even though the anti-CD8 antibody was infused less frequently than the anti-CD4 antibody.
CD4+ T-cell reconstitution after Ab-mediated CD4+ lymphocyte depletion in nonhuman primates. Peripheral blood (PB)– and lymph node (LN)–derived mononuclear cells were obtained from healthy SIV-uninfected sooty mangabeys (SMs) and rhesus macaques (RMs). (A) Flow cytometric staining of CD3+CD4+ T cells in PB of a representative SM (left panels) and RM (right panels) as measured before and at days 7 and 120 after Ab-mediated CD4+ lymphocyte depletion. (B) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the PB of SMs and RMs after CD4+ lymphocyte depletion. (C) Absolute number of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells in the PB of SMs and RMs after CD4+ lymphocyte depletion. (D) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the LN of SMs and RMs after CD4+ lymphocyte depletion. Values in panels B through D are means ± SD. SMs and RMs are shown as black squares and red triangles, respectively, and the dotted lines indicate the timing of anti-CD4 monoclonal antibody administrations.
CD4+ T-cell reconstitution after Ab-mediated CD4+ lymphocyte depletion in nonhuman primates. Peripheral blood (PB)– and lymph node (LN)–derived mononuclear cells were obtained from healthy SIV-uninfected sooty mangabeys (SMs) and rhesus macaques (RMs). (A) Flow cytometric staining of CD3+CD4+ T cells in PB of a representative SM (left panels) and RM (right panels) as measured before and at days 7 and 120 after Ab-mediated CD4+ lymphocyte depletion. (B) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the PB of SMs and RMs after CD4+ lymphocyte depletion. (C) Absolute number of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells in the PB of SMs and RMs after CD4+ lymphocyte depletion. (D) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the LN of SMs and RMs after CD4+ lymphocyte depletion. Values in panels B through D are means ± SD. SMs and RMs are shown as black squares and red triangles, respectively, and the dotted lines indicate the timing of anti-CD4 monoclonal antibody administrations.
CD8+ T-cell reconstitution after Ab-mediated CD8+ lymphocyte depletion in nonhuman primates. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Flow cytometric staining of CD3+CD8+ T cells in PB of a representative SM (left panels) and RM (right panels) before and at days 4 and 60 after Ab-mediated CD8+ lymphocyte depletion. (B) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the PB of SMs and RMs after CD8+ lymphocyte depletion. (C) Absolute number of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells in the PB of SMs and RMs after CD8+ lymphocyte depletion. (D) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the LN of SMs and RMs after CD8+ lymphocyte depletion. Values in panels B through D are means ± SD, SMs and RMs are shown as black squares and red triangles, respectively, and the dotted lines indicate the timing of anti-CD8 monoclonal antibody administrations.
CD8+ T-cell reconstitution after Ab-mediated CD8+ lymphocyte depletion in nonhuman primates. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Flow cytometric staining of CD3+CD8+ T cells in PB of a representative SM (left panels) and RM (right panels) before and at days 4 and 60 after Ab-mediated CD8+ lymphocyte depletion. (B) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the PB of SMs and RMs after CD8+ lymphocyte depletion. (C) Absolute number of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells in the PB of SMs and RMs after CD8+ lymphocyte depletion. (D) Fraction of CD3+CD4+ (left graph) and CD3+CD8+ (right graph) T cells, expressed as percentage variation from baseline, in the LN of SMs and RMs after CD8+ lymphocyte depletion. Values in panels B through D are means ± SD, SMs and RMs are shown as black squares and red triangles, respectively, and the dotted lines indicate the timing of anti-CD8 monoclonal antibody administrations.
Repopulation of CD4+ T cells after antibody-induced depletion is slow and occurs with similar kinetics in RMs and SMs
As described above, infusion of anti-CD4 antibody results in a similarly severe depletion of CD4+ T cells in both SMs and RMs. Given the different outcomes of SIV infection in these 2 species, we hypothesized that the AIDS-resistance of SMs is related to an intrinsically better ability to repopulate the CD4+ T-cell pool. To address this issue, we investigated the kinetics of CD4+ T-cell repopulation in SMs and RMs. As shown in Figure 1B-D, the kinetics of CD4+ T-cell repopulation after depletion in PB and LN were very similar in SMs and RMs. Importantly, the repopulation of CD4+ T cells was relatively slow and incomplete in both species, with the last available experimental time point showing a level of CD4+ T-cell reconstitution reaching only approximately 50% of baseline levels (Figure 1B-D).
Taken together, these findings suggest that an intrinsically reduced ability of CD4+ T cells to repopulate their pool after depletion may be involved in the HIV/SIV-associated CD4+ T-cell depletion. On the other hand, the observation of comparable kinetics of CD4+ T-cell reconstitution in RMs and SMs does not support the hypothesis that the AIDS resistance of SMs is caused by a more effective homeostatic response to the depletion of CD4+ T cells associated with SIV infection.
The repopulation of CD8+ T cells is more rapid than that of CD4+ T cells
We then investigated the kinetics of CD8+ T-cell repopulation after Ab-mediated CD8+ lymphocyte depletion. As shown in Figure 2, the repopulation of CD8+ T cells was relatively rapid in both species; already at day 11 after depletion, the fraction of CD3+CD8+ T cells in PB returned to 45.3% plus or minus 1.4% of baseline in SMs and to 50.6% plus or minus 14.1% of baseline in RMs. In terms of absolute numbers, we observed a significant increase of CD8+ T cells between day 11 and 21 in both species (Figure 2C). In LNs, the fraction of CD3+CD8+ T cells returned to 79.1% plus or minus 7.8% and 91.5% plus or minus 19.9% of baseline values in SMs and RMs, respectively (Figure 2D), a pattern of reconstitution considerably faster than that observed for CD4+ T cells (Figure 1). Interestingly, peripheral CD8+ T cells seem to repopulate similarly in the 2 studied species.
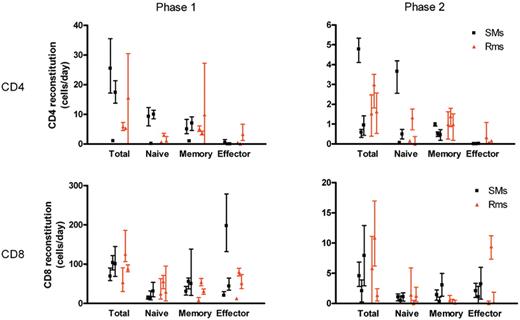
To compare the rates of T-cell reconstitution after depletion in SMs and RMs, we fitted the reconstitution data from individual monkeys to a bilinear model. This enabled us to compare the absolute rates of T-cell reconstitution (in cells/μL blood per day) between different T-cell subsets and monkeys. These fits revealed an initial rapid rate of reconstitution, and a second slower rate of reconstitution, where the initial rate of reconstitution may in part reflect the relatively rapid process of T-cell redistribution after depletion. These analyses reconfirmed that the CD8+ T-cell pool reconstitutes more quickly after depletion than the CD4+ T-cell pool. During the first rapid phase of reconstitution, CD8+ T cells increased in the blood at a median rate of 100 cells/μL blood per day in SMs and 88 cells/μL blood per day in RMs, compared with 17 CD4+ T cells/μL blood per day in SMs and 6 CD4+ T cells/μL blood per day in RMs. During the slower second phase CD8+ T cells also reconstituted more rapidly than CD4+ T cells: 4.6 and 5.8 CD8+ T cells/μL blood per day in SMs and RMs, respectively, compared with 0.6 and 1.5 CD4+ T cells/μL blood per day in SMs and RMs.
CD4+ and CD8+ T-cell repopulation after depletion involves both naive and memory T cells
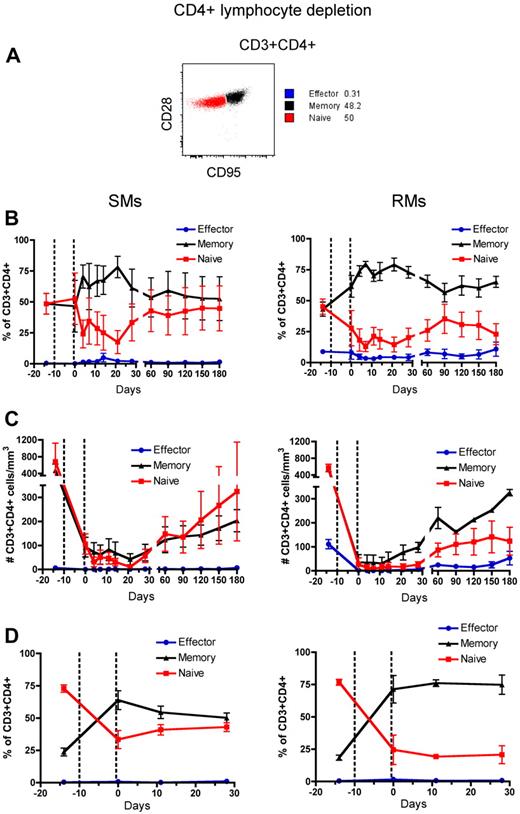
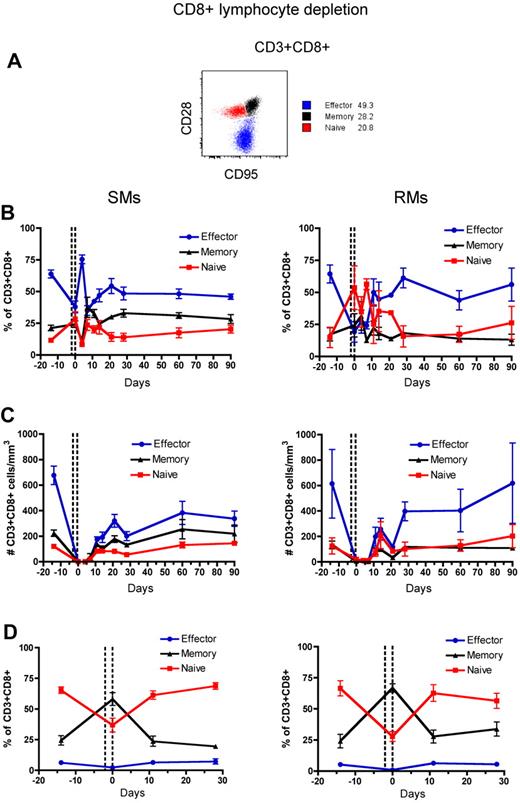
Naive and memory T-cell subsets of RMs and SMs can be classified based on the surface expression of CD28 and CD95, which allows to identify the naive (CD28+CD95−), memory (CD28+CD95+), and effector (CD28−CD95+) subsets of CD4+ and CD8+ T cells.37,38 To assess the phenotype of T cells that reconstitute the CD4+ and CD8+ T-cell compartments after depletion, we longitudinally measured the fraction of naive, memory, and effector CD4+ and CD8+ T cells in the blood and LN. The gating strategy is shown in Figures 3A and 4A, respectively. In both SMs and RMs, the average fraction of memory cells within the total CD4+ T-cell pool increased from approximately 50% (predepletion) to approximately 75% by day 21 after depletion (Figure 3B). This relative expansion of memory CD4+ T cells may reflect the fact that these cells were depleted less effectively than naive T cells (Figure 3C), and/or be caused by an intrinsically stronger proliferative potential of residual memory CD4+ T cells after depletion.39,40 In terms of absolute numbers, however, CD4+ T cells of SMs appear to recover both naive and memory subsets with similar rates, while CD4+ T cells of RMs show a more rapid reconstitution of the memory pool (Figure 3C). Indeed, in SMs the number of naive CD4+ T cells became similar to predepletion by day 120 after depletion, while in RMs this number remained significantly lower than baseline throughout the study (P < .01 for all time points). This latter finding suggests that SMs may be better able to replenish their naive CD4+ T-cell compartment. The depletion-induced enrichment in memory CD4+ T cells was more dramatic in LN, where the fraction of memory cells increased from approximately 20% predepletion to 60% and 75% at day 0 (ie, the last day of depleting treatment) in SMs and RMs, respectively (Figure 3D). Similarly to what we observed in blood, SMs tended to restore the predepletion proportion of naive and memory CD4+ T cells in LNs faster than RMs. Depletion of CD8+ lymphocytes was followed, in both species, by more subtle changes in the relative proportion of naive, memory, and effector CD8+ T cells, with the baseline proportion being restored by day 28 in blood and by day 11 in LNs (Figure 4B-D).
Naive, memory, and effector CD4+ T-cell reconstitution after Ab-mediated CD4+ lymphocyte depletion. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Gating strategies used to identify naive (red line), effector (blue line), and memory (black line) CD4+ T cells are shown in a representative animal. (B-D) Fractions (B, D) and absolute numbers (C) of naive, effector and memory cells in the CD4+ T-cell pool were longitudinally assessed after antibody-mediated depletion of CD4+ lymphocytes in the PB (B-C) or LN (D) of SMs (left graphs) and RMs (right graphs). Values in panels B through D are means ± SD, and the dotted lines indicate the timing of anti-CD4 monoclonal antibody administrations.
Naive, memory, and effector CD4+ T-cell reconstitution after Ab-mediated CD4+ lymphocyte depletion. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Gating strategies used to identify naive (red line), effector (blue line), and memory (black line) CD4+ T cells are shown in a representative animal. (B-D) Fractions (B, D) and absolute numbers (C) of naive, effector and memory cells in the CD4+ T-cell pool were longitudinally assessed after antibody-mediated depletion of CD4+ lymphocytes in the PB (B-C) or LN (D) of SMs (left graphs) and RMs (right graphs). Values in panels B through D are means ± SD, and the dotted lines indicate the timing of anti-CD4 monoclonal antibody administrations.
Naive, memory, and effector CD8+ T-cell reconstitution after Ab-mediated CD8+ lymphocyte depletion. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Gating strategies used to identify naive (red line), effector (blue line), and memory (black line) CD4+ T cells are shown in a representative animal. (B-D) Fractions (B,D) and absolute numbers (C) of naive, effector, and memory cells in the CD8+ T-cell pool were longitudinally assessed after antibody-mediated depletion of CD8+ lymphocytes in the PB (B-C) or LN (D) of SMs (left graphs) and RMs (right graphs). Values in panels B through D are means ± SD, and the dotted lines indicate the timing of anti-CD8 monoclonal antibody administrations.
Naive, memory, and effector CD8+ T-cell reconstitution after Ab-mediated CD8+ lymphocyte depletion. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Gating strategies used to identify naive (red line), effector (blue line), and memory (black line) CD4+ T cells are shown in a representative animal. (B-D) Fractions (B,D) and absolute numbers (C) of naive, effector, and memory cells in the CD8+ T-cell pool were longitudinally assessed after antibody-mediated depletion of CD8+ lymphocytes in the PB (B-C) or LN (D) of SMs (left graphs) and RMs (right graphs). Values in panels B through D are means ± SD, and the dotted lines indicate the timing of anti-CD8 monoclonal antibody administrations.
Fits of the reconstitution of the different T-cell subsets in individual animals demonstrated that the relative contribution of memory and naive T cells to both CD4+ and CD8+ T-cell reconstitution was similar, both in SMs and in RMs. With respect to CD4+ T cells, we found that during the initial rapid phase of reconstitution after CD4+ lymphocyte depletion, naive CD4+ T cells increased in the blood at a median rate of 9 cells/μL blood per day in SMs and 1.1 cells/μL blood per day in RMs, compared with memory CD4+ T cells which increased by 5 cells/μL blood per day in both SMs and RMs (Figure 5). The second slower phase of reconstitution also consisted of almost equal numbers of naive and memory CD4+ T cells: naive CD4+ T cells increased in the blood at a median rate of 0.5 cells/μL blood per day in SMs and 0.2 cells/μL blood per day in RMs, compared with memory CD4+ T cells which increased by 0.5 cells/μL blood per day in SMs and 1 cells/μL blood per day in RMs. In all, these findings indicate that naive and memory T cells contributed almost equally to the repopulation of the CD4+ and CD8+ T-cell pools.
Quantitative kinetics of T-cell subset reconstitution after Ab-mediated CD4+ or CD8+ lymphocyte depletion. The data on the reconstitution of total CD4+ (A) or CD8+ (B) T-cell pools, as well as their naive, memory, and effector subsets in individual SMs and RMs, were fitted to a bilinear model. In this model, an initial rapid phase (phase 1 left graphs) and a second slower phase (phase 2 right graphs) of increase of cell numbers was distinguished. The levels of T-cell reconstitution are graphed as cells/μL blood/day for each of the 3 uninfected SMs and 3 uninfected RMs included in the study, with bars representing the 95% confidence interval of the estimated parameter.
Quantitative kinetics of T-cell subset reconstitution after Ab-mediated CD4+ or CD8+ lymphocyte depletion. The data on the reconstitution of total CD4+ (A) or CD8+ (B) T-cell pools, as well as their naive, memory, and effector subsets in individual SMs and RMs, were fitted to a bilinear model. In this model, an initial rapid phase (phase 1 left graphs) and a second slower phase (phase 2 right graphs) of increase of cell numbers was distinguished. The levels of T-cell reconstitution are graphed as cells/μL blood/day for each of the 3 uninfected SMs and 3 uninfected RMs included in the study, with bars representing the 95% confidence interval of the estimated parameter.
Ab-mediated CD4+ and CD8+ lymphocyte depletion in SMs and RMs is followed by a rapid, largely lineage-specific increase in T-cell proliferation
To determine how the depletion of CD4+ and CD8+ lymphocytes influences the proliferation of the residual T cells in RMs and SMs, we longitudinally assessed the fraction of these cells expressing the proliferation marker Ki-67 in both PB and LN. We found that, in both SMs and RMs, the depletion of CD4+ T cells was followed by a dramatic increase of their levels of proliferation, whose kinetics were similar (no significant difference at any time point post depletion) in the 2 species (Figure 6A). Indeed, in both species, the fraction of CD4+Ki-67+ T cells was 3 times higher compared with predepletion levels by day 4 after depletion, and remained higher until day 28 after depletion (Figure 6A). Consistent with the slow and incomplete reconstitution of CD4+ T cells (Figure 1), which may have triggered a prolonged homeostatic response, CD4+ T-cell proliferation remained higher than baseline throughout the follow-up period in both species (Figure 6A). A similar albeit less dramatic increase in the levels of proliferating CD4+ T cells was found in the LNs of the CD4+ lymphocyte depleted animals (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We next analyzed the levels of memory CD4+ T-cell proliferation in the blood. As shown in Figure 6B, in both RMs and SMs the depletion of CD4+ lymphocytes was followed by a rapid (ie, starting at day 4 after depletion) increase in the memory CD4+ T-cell proliferation, with comparable kinetics in the 2 species. Of note, in both species the fraction of memory CD4+Ki67+ T cells returned to predepletion levels by day 60 after depletion (Figure 6B). This increase in memory CD4+ T-cell proliferation was specific for circulating lymphocytes, since no changes were found in the LNs of the same animals (Figure 6B).
T-cell proliferation after Ab-mediated CD4+ and CD8+ lymphocyte depletion in nonhuman primates. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Levels of circulating CD4+ (left graphs) and CD8+ (right graphs) T cells of SMs (black line) and RMs (red line) that express the proliferation marker Ki-67 after Ab-mediated CD4+ lymphocyte depletion. (B) Levels of memory (ie, CD28+CD95+) CD4+ T cells that express Ki-67 in the PB (left graph) or LN (right graph) of SMs (black line) and RMs (red line) after Ab-mediated CD4+ lymphocyte depletion. (C) Levels of circulating CD4+ (left graphs) and CD8+ (right graphs) T cells of SMs (black line) and RMs (red line) that express Ki-67 after Ab-mediated CD8+ lymphocyte depletion. In all the graphs levels of Ki-67+ T cells are shown as percentage variation from baseline (mean ± SD), and the dotted lines indicate the timing of anti-CD4 (A-B) or anti-CD8 (C) monoclonal antibody administrations.
T-cell proliferation after Ab-mediated CD4+ and CD8+ lymphocyte depletion in nonhuman primates. PB- and LN-derived mononuclear cells were obtained from healthy SIV-uninfected SMs and RMs. (A) Levels of circulating CD4+ (left graphs) and CD8+ (right graphs) T cells of SMs (black line) and RMs (red line) that express the proliferation marker Ki-67 after Ab-mediated CD4+ lymphocyte depletion. (B) Levels of memory (ie, CD28+CD95+) CD4+ T cells that express Ki-67 in the PB (left graph) or LN (right graph) of SMs (black line) and RMs (red line) after Ab-mediated CD4+ lymphocyte depletion. (C) Levels of circulating CD4+ (left graphs) and CD8+ (right graphs) T cells of SMs (black line) and RMs (red line) that express Ki-67 after Ab-mediated CD8+ lymphocyte depletion. In all the graphs levels of Ki-67+ T cells are shown as percentage variation from baseline (mean ± SD), and the dotted lines indicate the timing of anti-CD4 (A-B) or anti-CD8 (C) monoclonal antibody administrations.
We next analyzed the proliferative CD8+ T-cell response in CD8+ lymphocyte depleted animals and found that, in both species, the Ab-mediated depletion was followed by a rapid increase in the fraction of circulating CD8+Ki-67+ T cells (Figure 6C). At day 7 after depletion, this proliferative response was much higher in SMs compared with RMs: PB-derived CD8+ T cells of CD8+ lymphocyte depleted SMs expressed 10 times higher levels of Ki-67 than predepletion, while in RMs the increase was only 2.3 fold (Figure 6C). As observed for CD4+ T-cell proliferation, the increase in CD8+ T-cell proliferation was more limited in LN, with the fraction of CD8+Ki-67+ cells being slightly increased only at day 0 after depletion (supplemental Figure 1B). As expected based on the fast reconstitution of the peripheral CD8+ T-cell compartment, the fraction of proliferating CD8+Ki-67+ T cells returned to predepletion levels in both species by day 11 after depletion and in both blood (Figure 6C) and LN (Supp. Figure 1B). Of note, in both SMs and RMs, the proliferation that followed CD4+ or CD8+ lymphocyte depletion was predominantly lineage-specific, with an increase in CD4+ T-cell proliferation and only limited changes in CD8+ T-cell proliferation when CD4+ lymphocytes were depleted (Figure 6A), and an increase in CD8+ T-cell proliferation with only limited changes in CD4+ T-cell proliferation (particularly so for SMs) when CD8+ lymphocytes were depleted (Figure 6C).
Taken together, these data indicate that the increased level of proliferation that follows a rapid and severe depletion of CD4+ or CD8+ lymphocytes is quantitatively similar in RMs and SMs, involves by-and-large lineage-specific circulating T cells, and appears to be shaped by the ongoing level of CD4+ or CD8+ T-cell reconstitution.
CD4+ and CD8+ lymphocyte depletions are followed by an early plasmatic increase of interleukin-7 (IL-7)
Cytokines such as interleukin-7 (IL-7) and interleukin-15 (IL-15) play a critical role in promoting T cell homeostasis.7–11,41 To investigate the role of IL-7 and IL-15 in the reconstitution of CD4+ and CD8+ T cells that follows antibody-mediated depletion, we longitudinally assessed the plasma levels of these cytokines. Consistent with previous studies,34 baseline (ie, predepletion) plasma levels of IL-7 and IL-15 were higher in RMs (2.36 ± 1.5 pg/mL and 7.54 ± 0.1 pg/mL, respectively) than in SMs (0.30 ± 0.1 pg/mL and 0.65 ± 0.3 pg/mL, respectively). To account for these different baseline levels, we chose to present plasma levels of IL-7 and IL-15 as a percentage of their predepletion values.
As shown in Figure 7A-B, in both species plasma levels of IL-7 increased rapidly after CD4+ (Figure 7A) and CD8+ (Figure 7B) lymphocyte depletion. Interestingly, in RMs plasma IL-7 levels after CD4+ lymphocyte depletion remained significantly higher than baseline throughout the follow up period, while in SMs they returned to predepletion levels by day 60 despite an incomplete reconstitution of the CD4+ T-cell compartment. Of note, in SMs the restoration of baseline levels of IL-7 by day 60 after depletion temporally correlated with the restoration of predepletion levels of both proliferating memory CD4+ T cells (Figure 6B) and the frequency of memory CD4+ T cells (Figure 3B). In contrast, the kinetics of plasma IL-7 levels after CD8+ lymphocyte depletion were very similar in SMs and RMs, with IL-7 remaining significantly higher compared with baseline throughout the follow-up period (Figure 7B). Finally, hardly any significant changes were observed in plasma IL-15 levels after CD4+ or CD8+ lymphocyte depletion, with the exception of a late (ie, starting at day 60) increase of IL-15 levels in SMs after CD4+ lymphocyte depletion (Figure 7A-B).
Variation of IL-7 and IL-15 plasma levels as well as IL-7 receptor (CD127) expression after Ab-mediated CD4+ and CD8+ lymphocyte depletion in nonhuman primates. (A-B) Plasmas isolated from healthy SIV-uninfected SMs and RMs were tested for levels of IL-7 (black line) and IL-15 (red line). Levels of these cytokines are expressed as percentage variation from baseline (mean ± SD) in SMs (left graphs) and RMs (right graphs) after Ab-mediated CD4+ (A) and CD8+ (B) lymphocyte depletion. (C) Correlation between change over time (Δ; ie, percentage variation compared with baseline) of plasma IL-7 and those of proliferating CD4+Ki67+ T cells. (D) Levels of CD4+CD127+ T cells (expressed as percentage variation from baseline) in SMs (black line) and RMs (red line) after Ab-mediated CD4+ lymphocyte depletion. The dotted lines in the graphs indicate the timing of anti-CD4 monoclonal antibody administrations.
Variation of IL-7 and IL-15 plasma levels as well as IL-7 receptor (CD127) expression after Ab-mediated CD4+ and CD8+ lymphocyte depletion in nonhuman primates. (A-B) Plasmas isolated from healthy SIV-uninfected SMs and RMs were tested for levels of IL-7 (black line) and IL-15 (red line). Levels of these cytokines are expressed as percentage variation from baseline (mean ± SD) in SMs (left graphs) and RMs (right graphs) after Ab-mediated CD4+ (A) and CD8+ (B) lymphocyte depletion. (C) Correlation between change over time (Δ; ie, percentage variation compared with baseline) of plasma IL-7 and those of proliferating CD4+Ki67+ T cells. (D) Levels of CD4+CD127+ T cells (expressed as percentage variation from baseline) in SMs (black line) and RMs (red line) after Ab-mediated CD4+ lymphocyte depletion. The dotted lines in the graphs indicate the timing of anti-CD4 monoclonal antibody administrations.
To further elucidate the role of IL-7 in CD4+ T-cell homeostasis of nonhuman primates, we next investigated the relationship between plasma levels of IL-7 and either CD4+ T-cell counts or the fraction of proliferating (ie, Ki-67+) CD4+ T cells. We found that changes of plasma IL-7 (ΔIL-7, ie, percentage variation compared with baseline) correlated inversely with the changes of CD4+ T-cell counts (P < .001, data not shown) and directly with the changes in the fraction of CD4+Ki-67+ T cells (P = .007, Figure 7C). These findings suggest a role of IL-7 in regulating CD4+ T-cell homeostatic proliferation, and are consistent with 2 recent published studies showing a beneficial effect of IL-7 in inducing CD4+ T-cell proliferation and reconstitution in HIV-infected humans.42,43 The moderate and transient increase in the levels of proliferation of the nondepleted subsets (see Figure 6) may also be related to the increased availability of circulating IL-7 after CD4+ or CD8+ lymphocyte depletion.
Finally, we longitudinally assessed the levels of CD127, that is, the α-chain of the IL-7 receptor, on CD4+ T cells of the CD4+ lymphocyte depleted animals. In both SMs and RMs, the depletion of CD4+ T cells and the concomitant increase in plasma levels of IL-7 were followed by a rapid decrease in the fraction of CD4+ T cells expressing CD127 (Figure 7D). While the kinetics of CD127 expression was similar in the 2 species, the observed fluctuations were slightly higher in RMs (nadir value after depletion was 42.4 ± 6.2% of baseline levels) than SMs (nadir value after depletion was 67.6 ± 7.7% of baseline levels). These findings suggest that, in both SMs and RMs, IL-7–dependent mechanisms may play an important role in determining the increased levels of CD4+ or CD8+ T-cell proliferation that follow the antibody-mediated depletion of these cells.
Discussion
Despite the crucial role of CD4+ and CD8+ T cells in maintaining immune function, the mechanisms regulating their homeostasis in vivo are still poorly understood, and particularly so in primates, where inbred transgenic/knock out animal strains are not available. This relative lack of knowledge has a negative impact on our understanding of the pathogenesis of HIV infection,13 in which CD4+ T cells are depleted during chronic infection. Basic unanswered questions regarding T-cell homeostasis in primates include: (1) Is the homeostasis regulated similarly between CD4+ and CD8+ T cells? (2) is the homeostasis of the naive, memory and effector T-cell subsets regulated in the same way? (3) Is the homeostatic proliferation of CD4+ and CD8+ T cells lineage-specific? (4) What molecular mechanisms and anatomic compartments support the homeostatic proliferation of T cells? In the context of AIDS pathogenesis, an additional key question is whether CD4+ T-cell homeostasis is regulated in a different way in species that experience pathogenic HIV or SIV infections (ie, humans and RMs) versus species that experience a nonprogressive SIV infection (ie, SMs).26 This last question is important because elucidating the mechanisms responsible for the preservation of CD4+ T-cell homeostasis in natural hosts for SIV may help developing therapeutic approaches aimed at preventing or treating CD4+ T-cell depletion in HIV-infected humans.
In this study, we conducted experiments of CD4+ or CD8+ lymphocytes depletion in nonhuman primates. We selected 2 species that experience either progressive (RMs) or nonprogressive (SMs) SIV infection, to test the hypothesis that the AIDS-resistance of SMs may be related to a species-specific, genetically determined ability to preserve CD4+ T-cell homeostasis in the presence of a depleting event. To the best of our knowledge, this is the first time that a comparative, longitudinal analysis of CD4+ and CD8+ T-cell repopulation after in vivo depletion has been performed in these 2 species of nonhuman primates. The main results of this study are the following: (1) in both RMs and SMs, the depleting antibodies induced a rapid, profound, and largely specific (ie, without impacting the nontargeted populations) depletion of CD4+ or CD8+ T cells; (2) in both species, CD4+ and CD8+ lymphocyte depletions were followed by a rapid proliferation which was largely lineage specific, ie, involving predominantly the depleted subset of T cells; (3) in both species, the reconstitution of CD4+ T cells was significantly slower than that of CD8+ T cells, even though the used antibodies were more effectively depleting CD8+ than CD4+ T lymphocytes; and (4) in both species, CD4+ and CD8+ lymphocyte depletion was followed by increased plasma levels of IL-7 which, in turn, correlated with increased proliferation of the depleted T-cell subset. In addition, the increase in plasma levels of IL-7 that followed CD4+ lymphocyte depletion was associated with reduced expression of CD127 (IL-7R) on CD4+ T cells.
These data indicate that the kinetics of CD4+ and CD8+ T-cell reconstitution after depletion are relatively similar in SMs and RMs, therefore not supporting the hypothesis that SIV-infected SMs maintain healthy CD4+ T-cell counts due to an intrinsically more rapid homeostatic response to depletion. Nonetheless, an interesting qualitative difference between SMs and RMs in CD4+ T-cell repopulation was that only the former were able to recover to the baseline ratio of naive and memory cells. It is tempting to speculate that this ability of SMs to better reconstitute a naive-rich CD4+ T-cell compartment may help protect these animals when infected with SIV. However, the antibody-mediated depletion of CD4+ lymphocytes is clearly different in many ways from that induced in vivo by SIV infection. For instance, the antibody-mediated depletion is more rapid, involves virtually all CD4+ T-cell subsets, and based on other studies,30 involves predominantly CD4+ T cells in the blood and LN. In contrast, the SIV-induced depletion is slower, involves predominantly CD4+CCR5+ T cells, and affects mucosal tissues more than blood and LN.44,45 For these reasons, the hypothesis that a more effective preservation of CD4+ T-cell homeostasis plays a role in the AIDS resistance of naturally SIV-infected SMs (and possibly other natural SIV hosts) needs to be tested with further and more targeted interventions.
Despite the relatively low number of animals included in each experimental setting, the current study provides useful information regarding the basic features of CD4+ and CD8+ T-cell homeostasis in nonhuman primates, which is an understudied field of research. First, our study indicated clearly that, in both SMs an RMs, the CD4+ T-cell repopulation after antibody-mediated depletion is significantly less efficient compared with that of CD8+ T cells. In addition, CD8+ T cells reconstitute more rapidly the predepletion ratio of naive and memory cells. These observations are consistent with the fact that, in humans undergoing hematopoietic stem cell transplantation, CD4+ T-cell reconstitution is much slower than that of CD8+ T cells.46,47 The possibility that CD4+ T-cell homeostasis is intrinsically more fragile than that of CD8+ T cells is compatible with the notion that the immunologic damage caused by the chronic immune activation of pathogenic HIV/SIV infections affects CD4+ T cells more than the CD8+ T cells, above and beyond the fact that these viruses preferentially infect and kill CD4+ T cells.14 Second, our study indicates that the homeostatic proliferation of T cells which follows antibody-mediated depletion is by-and-large lineage-specific, ie higher in the depleted compared with the nondepleted compartments; this is particularly evident in the setting of CD4+ lymphocyte depletion. This finding does not support the model, originated from mouse studies, that T-cell homeostasis is blind to the distinction between CD4+ and CD8+ T cells.48–50 The current study emphasizes our limited understanding of the molecular mechanisms underlying the lineage specificity of the T-cell proliferation that follows antibody-mediated depletion. Interestingly, our results are consistent with the observation that in idiopathic CD4 lymphopenia (ICL), a rare syndrome associated with low CD4+ T-cell counts in absence of HIV, only CD4+ but not CD8+ T cells are activated and cycling.51 In contrast to ICL,52 however, antibody-mediated CD4+ (or CD8+) lymphocyte depletion is not associated with increased microbial translocation (as assessed by sCD14 levels) at any of the studied experimental points in either species (data not shown). Third, our study indicates that, in both species, CD4+ and CD8+ lymphocyte depletion is followed by a rapid increase in plasma IL-7, coincident with the peak of homeostatic T-cell proliferation. This finding suggests that, in healthy nonhuman primates, IL-7 plays an important role in the homeostatic response to the experimental depletion of CD4+ and CD8+ T cells. Interestingly, the increase of plasma IL-7 that follows CD4+ lymphocyte depletion was more prolonged in RMs than in SMs, even in the setting of a similarly incomplete reconstitution of the CD4+ T-cell pool. This pattern was observed previously after the acute phase of SIV infection during the initial early decline in CD4+ T-cell counts which is associated with increased levels of plasma IL-7 and proliferating T cells.34,53 The similarity between antibody-mediated depletion and acute SIV infection was further indicated as the increases in plasma IL-7 and T-cell proliferation were lower and more transient in SMs compared with the RMs.34,53 The fact that SMs may have a more attenuated homeostatic response to CD4+ T-cell depletion could, paradoxically, facilitate their ability to preserve CD4+ T-cell homeostasis during chronic SIV infection, especially since proliferating CD4+ T cells may serve as targets for SIV replication.
In conclusion, this analysis of the kinetics of CD4+ and CD8+ T-cell repopulation after antibody-mediated depletion provided important preliminary information on the regulation of T-cell homeostasis in nonhuman primates. Further studies of T-cell homeostasis in nonhuman primate species that experience either a progressive or a nonprogressive SIV infection may improve our basic understanding of AIDS pathogenesis and help in designing therapeutic intervention aimed at preserving CD4+ T-cell homeostasis in HIV-infected individuals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Silvija Staprans for helpful discussions, Stephanie Ehnert and Dr Elizabeth Strobert for their assistance with animal studies, and Sarah Ratcliffe for her assistance in the statistical analysis of the data.
This work was supported by grants R21-AI-54 234 and R01-HL075766 (to G.S).
National Institutes of Health
Authorship
Contribution: M.P., B.C., and G.S. designed the study and wrote the paper, with contributions from the other authors as appropriate; J.C.E. and B.C. performed the immunophenotypic analyses and prepared the figures; J.A.M.B. and R.J.B. fitted experimental data to a mathematical model and contributed to the writing of the paper; S.N.G., A.C., and N.R.K. helped in processing the samples and in analyzing the results; N.R.K. and J.M.B. provided the data on dendritic cells and sCD14; R.S.M. provided the depleting antibodies; J.E. supervised the housing and care of the animals and contributed to the design of the study; and D.L.S. provided the IL-7 and IL-15 data and contributed to the design of the study.
The current affiliation for B.C. and M.P. is Yerkes National Primate Research Center, Emory University, Atlanta, GA.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mirko Paiardini, PhD, Emory University, Yerkes National Primate Research Center, 954 Gatewood Rd NE, Atlanta, GA 30329; e-mail: mirko.paiardini@emory.edu.
References
Author notes
J.C.E. and B.C. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal