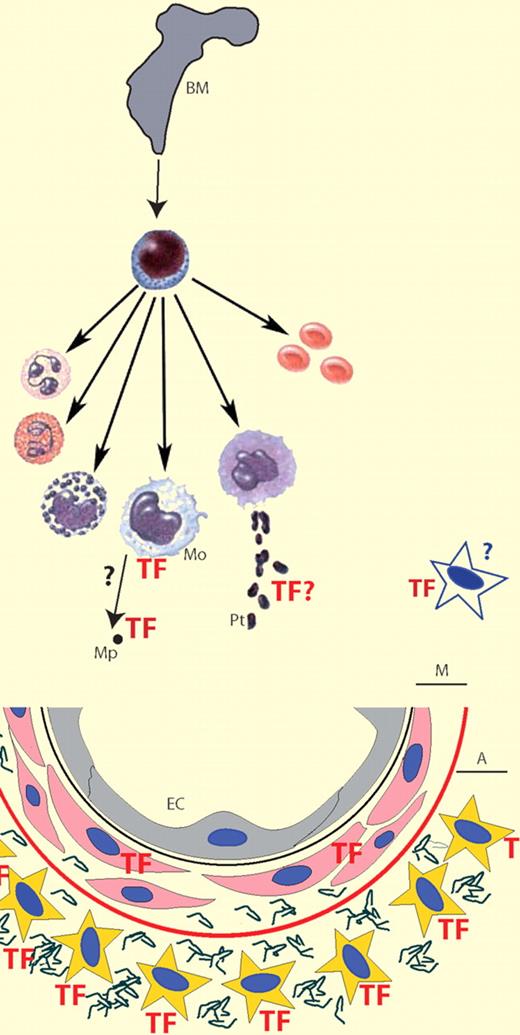
TF, the initiator of coagulation, is normally not expressed by cells within the vasculature; rather, it is found in the medial (M) and adventitial (A) layers around blood vessels. Endotoxemia induces monocyte (Mo) TF expression that contributes significantly (∼ 60%) to the procoagulant response. In mice, platelet (Pt) TF expression is not relevant in this model; however, MP-associated TF does contribute. Endothelial cells and vascular smooth muscle cells do not contribute but an unidentified nonhemopoetic cell type(s) contributes significantly (∼ 40%) to the procoagulant response.
TF, the initiator of coagulation, is normally not expressed by cells within the vasculature; rather, it is found in the medial (M) and adventitial (A) layers around blood vessels. Endotoxemia induces monocyte (Mo) TF expression that contributes significantly (∼ 60%) to the procoagulant response. In mice, platelet (Pt) TF expression is not relevant in this model; however, MP-associated TF does contribute. Endothelial cells and vascular smooth muscle cells do not contribute but an unidentified nonhemopoetic cell type(s) contributes significantly (∼ 40%) to the procoagulant response.
The blood coagulation system is normally initiated in response to injury to preserve the integrity of the vascular system. The ability to stem the loss of body fluids from the site of injury is a basic defense mechanism that is essential for the survival of any multicellular organism. The critical need to rapidly form a stable, localized clot in response to injury must be balanced with the need to maintain blood flow within the vessel. The initiation of coagulation occurs when blood is exposed to cells expressing tissue factor (TF). The anatomical distribution of cells constitutively expressing TF has led to the concept that TF forms a hemostatic envelope surrounding blood vessels, organs, and the organism itself. TF is present in medial and adventitial cells including vascular smooth muscle cells (VSMCs), fibroblasts, and pericytes of the blood vessel and serves to limit blood loss after vascular injury. In contrast, cells within the vasculature including endothelial cells (ECs) do not normally express TF, thus maintaining blood flow by presenting an anticoagulant surface. More recently, the identification of “blood borne” TF either on circulating microparticles (MPs) or as a soluble protein, and the possibility that platelets may express TF, has challenged this concept. However, there are contradictory reports about the synthesis and presentation of TF on circulating blood cells and the presence or absence of functionally active TF circulating in blood (see figure).
In endotoxemia, lipopolysaccharide (LPS) induces expression of TF on monocytes and it was believed that this was the major source of TF that contributes to disseminated intravascular coagulation. However, several cell types that come into contact with blood have been shown to synthesize TF in response to a variety of different agonists including LPS. The relative contribution of TF from various cell types to the activation of coagulation in endotoxemia is unknown. Furthermore, although the response of both monocytes and ECs in vitro has been well documented and shown to be very similar, evidence for EC expression of TF in vivo is scant.
In the present study Pawlinski et al investigate the relative contributions of various cell types in a mouse model of endotoxemia using both inhibitory antibodies to inhibit TF-mediated coagulation and a series of cell lineage–specific TF gene deletion transgenic mice.1 Using a well-established mouse model in which animals receive a single intraperitoneal injection of LPS, the authors have measured plasma concentrations of thrombin-antithrombin (TAT) complex 8 hours after LPS injection as a surrogate marker of blood coagulation activation. Administration of an inhibitory anti-TF antibody before LPS administration significantly reduced TAT levels. Furthermore, using bone marrow reconstitution they were able to generate mice that showed that hemopoietic and nonhematopoietic cells contribute approximately 60% and 40%, respectively, of the TF-dependent activation of coagulation in response to LPS. To determine the hemopoietic cells expressing TF, they generated mice in which the TF gene was effectively deleted in cells that express a myeloid-specific gene. Inactivation of myeloid TF expression reduced TAT levels to those generated by nonhemopoietic cells, indicating that myeloid cells primarily contribute to the hemopoietic TF-dependent procoagulant response. It was not possible to determine which cell type within the myeloid lineage is the source of TF because the TF gene deletion occurs in monocytes and neutrophils. While TF expression by monocytes in response to LPS is well established and is the likely source of most of the TF activity, expression of TF by granulocytes is controversial, with some authors reporting expression of TF in neutrophils and eosinophils whereas others show no evidence of TF expression on granulocytes.2,–4
TF expression in platelets is also controversial with different mechanisms of platelet TF expression described5,,,–9 : translocation and activation of preexisting TF from intracellular compartments, uptake of TF from other sources such as MP, and de novo TF mRNA and protein synthesis. Human platelets have been shown to contain precursor-mRNA (pre-mRNA) encoding TF, and in response to different agonists the mRNA is processed into a mature translatable TF mRNA. To address the contribution of platelet TF in this mouse model of endotoxemia, the authors analyzed TF mRNA and showed that LPS-stimulated mouse platelets do not express either pre-mRNA or mature TF mRNA indicating apparent species differences in platelet TF expression. Furthermore, deletion of the TF gene in megakaryocytes did not affect plasma TAT levels in LPS-treated mice. The data suggest that platelet expression of TF does not contribute to the procoagulant response in this mouse model, although it does not exclude a role for platelets in de-encrypting MP-associated TF. Indeed, LPS increases the level of TF-bearing MPs in mice that correlates well with plasma TAT levels.10
ECs and VSMCs were strong candidates for the nonhematopoietic cells expressing TF because ECs have been shown in vitro to respond to LPS stimulation in an identical manner to monocytes, but controversially there is very little evidence for TF expression by ECs in vivo. Alternatively, because endotoxemia is associated with increased vascular permeability this could expose TF-positive VSMCs and contribute to the procoagulant response. In another series of cell type–specific TF gene deletion experiments, Pawlinski et al elegantly demonstrate that neither EC nor VSMC TF expression contributes significantly to the procoagulant response observed at 8 hours after LPS treatment.
This stimulating manuscript contributes substantially to this controversial area, furthering our understanding of the cells that initiate intravascular coagulation. This study has used a mouse model of endotoxemia and examined only a single time point. It will now be of interest to see whether these results can be replicated in other models to determine whether the relative contribution of different cell types changes during the response. Finally, what is the identity of the nonhemopoietic cell(s) that contribute substantially to the TF-mediated procoagulant response?
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal