Abstract
Studies in mice have shown that proinflammatory Th17 cells can cause acute graft-versus-host disease (aGVHD) related tissue damage; however, whether they play a role in human aGVHD remains unclear. In a prospective study, we measured the proportion of Th17 cells in the blood and skin of patients at the onset of aGVHD. We found no difference in the proportion or amount of IL-17 produced by T cells in the blood of patients with aGVHD (n = 20) compared with time-matched patients without GVHD (n = 14). Moreover, Th17 cells were not increased in the skin of patients with cutaneous aGVHD (n = 7) compared with healthy controls (n = 10). In contrast, we found significantly more interferon-γ–producing T cells in the skin of patients with aGVHD compared with controls. These data support the long-standing paradigm that tissue localized interferon-γ–producing cells are the perpetrators of aGVHD.
Introduction
The acute form of graft-versus-host disease (aGVHD) is classically thought to be mediated by Th1 cells.1 This paradigm is controversial, however, because aGVHD can be induced by Th2 cells, and Stat4- or interferon-γ (IFN-γ)-deficient T cells.2,3 The identification of proinflammatory Th17 cells, defined by production of interleukin-17 (IL-17) and IL-22,4 led to speculation that Th17 cells may have an unrecognized role in GVHD. Studies in IL-17−/− mice found conflicting results, with some data suggesting that IL-17 is protective5 and others indicating a pathogenic role.6,7 In support of the latter studies, transplantation of Th17 cells causes GVHD-related tissue damage.8 In humans, there are also divergent results with retrospective reports that circulating and/or tissue localized Th17 cells can be increased9 or decreased10 in GVHD; hence, their role in GVHD pathogenesis remains unclear. We performed a prospective study in patients undergoing allogeneic stem cell transplantation and asked whether altered proportions of circulating and/or tissue localized Th17 cells correlated with the onset of aGVHD.
Methods
Study population
Peripheral blood samples were collected weekly and at onset of aGVHD from 50 allogeneic stem cell transplantation patients (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We analyzed samples from patients at onset of aGVHD (GVHD+; n = 20) and time-matched samples from patients without GVHD (GHVD−; n = 14). Twenty age-matched healthy donors served as controls. Patients with veno-occlusive disease, periengraftment syndrome, or septic shock were excluded from the study (n = 16). aGVHD was confirmed by biopsy and staged per modified Gluckberg criteria.11 The study was approved by the University of British Columbia Clinical Research Ethics Board.
Cytokine analysis
Details of cytokine detection are given in the supplemental data.
Results and discussion
Circulating T cells from aGVHD patients do not have elevated IL-17 production
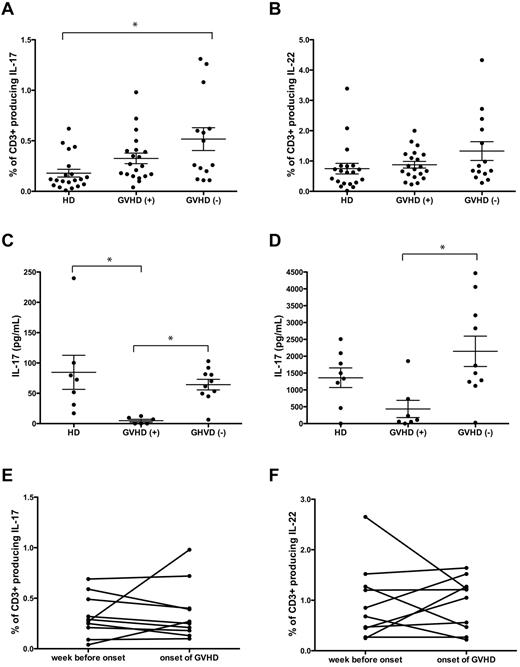
Although in mice Th17 cells traffic to GVHD target organs and mediate lethal GVHD,7,8 whether Th17 cells contribute to the pathogenesis of GVHD in humans remained unclear. Because IL-17–producing T cells may be CD4+ or CD8+,12 the frequency of IL-17–, IL-22–, and IFN-γ–producing CD3+ T cells was compared in GVHD+ versus GVHD− allogeneic stem cell transplantation patients. Our prospective design allowed us to analyze the frequency of Th17 cells at the onset of aGVHD before treatment with steroids. The frequency of IL-17+ CD3+ T cells from GVHD− patients did not differ from that in GHVD− patients but was significantly higher than in healthy donors (Figure 1A; supplemental Figure 1). There was no difference in the proportion of IL-22– or IFN-γ–producing CD3+ T cells between GVHD+ patients, GVHD− patients, or healthy donors (Figure 1B and data not shown).
The onset of aGVHD is not correlated with an increase in circulating Th17 cells. PBMCs from transplanted patients were collected at the onset of GVHD before commencing steroid therapy (GVHD+; n = 20) and one week before onset of GVHD (n = 10). Healthy donors (HDs; n = 20) and transplanted patients without GVHD (n = 14) served as controls (GVHD−). PBMCs were stimulated with phorbol myristate acetate and ionomycin, and gated CD3+ T cells were analyzed for production of (A) IL-17 (GVHD+, 0.33% ± 0.05%; GVHD−, 0.52% ± 0.11%; healthy donors, 0.18% ± 0.04%) and (B) IL-22 (GVHD+, 0.88% ± 0.11%; GVHD−, 1.33% ± 0.31%; healthy donors, 0.75% ± 0.17%.). Supernatants from PBMCs activated via αCD3/CD28 were analyzed for production of (C) IL-17 (GVHD+, 4.83 ± 2.28 pg/mL, n = 7; GVHD−, 64.3 ± 8.76 pg/mL, n = 10; healthy donors, 84.55 ± 28.05 pg/mL, n = 8) and (D) IL-22 (GVHD+, 433 ± 254 pg/mL, n = 7; GVHD− patients, 2146 ± 451 pg/mL, n = 10; and healthy donors 1361.0 ± 209.7 pg/mL, n = 8). Amounts of (E) IL-17 and (F) IL-22 secreted by phorbol myristate acetate and ionomycin stimulated PBMCs isolated one week before the onset of GHVD was compared with production at onset (P = not significant). Each spot represents an individual donor/patient. Error bars represent SEM. *P < .05.
The onset of aGVHD is not correlated with an increase in circulating Th17 cells. PBMCs from transplanted patients were collected at the onset of GVHD before commencing steroid therapy (GVHD+; n = 20) and one week before onset of GVHD (n = 10). Healthy donors (HDs; n = 20) and transplanted patients without GVHD (n = 14) served as controls (GVHD−). PBMCs were stimulated with phorbol myristate acetate and ionomycin, and gated CD3+ T cells were analyzed for production of (A) IL-17 (GVHD+, 0.33% ± 0.05%; GVHD−, 0.52% ± 0.11%; healthy donors, 0.18% ± 0.04%) and (B) IL-22 (GVHD+, 0.88% ± 0.11%; GVHD−, 1.33% ± 0.31%; healthy donors, 0.75% ± 0.17%.). Supernatants from PBMCs activated via αCD3/CD28 were analyzed for production of (C) IL-17 (GVHD+, 4.83 ± 2.28 pg/mL, n = 7; GVHD−, 64.3 ± 8.76 pg/mL, n = 10; healthy donors, 84.55 ± 28.05 pg/mL, n = 8) and (D) IL-22 (GVHD+, 433 ± 254 pg/mL, n = 7; GVHD− patients, 2146 ± 451 pg/mL, n = 10; and healthy donors 1361.0 ± 209.7 pg/mL, n = 8). Amounts of (E) IL-17 and (F) IL-22 secreted by phorbol myristate acetate and ionomycin stimulated PBMCs isolated one week before the onset of GHVD was compared with production at onset (P = not significant). Each spot represents an individual donor/patient. Error bars represent SEM. *P < .05.
The low frequency of IL-17–producing cells detected by intracellular staining may result in poor sensitivity to detect differences in cytokine production; therefore, amounts of IL-17 and IL-22 in the supernatants from peripheral blood mononuclear cells (PBMCs) activated via αCD3/CD28 were measured. In contrast to intracellular cytokine data where we found no difference between GVHD+ and GVHD− patients, the levels of IL-17 were significantly decreased in GVHD+ patients compared with GVHD− patients (Figure 1C). In keeping with decreased IL-17 production, lower levels of IL-22 were detected in GVHD+ compared with GVHD− patients (Figure 1D). The proportions of PBMCs that were CD3+ T cells did not differ between the 3 groups (data not shown).
We considered the possibility that circulating IL-17+ cells may be involved in the early stage of GVHD pathogenesis but that at aGVHD onset they may exit the circulation, possibly trafficking to the inflamed tissue. Thus, in a subset of patients, we analyzed samples taken one week before the onset of GVHD. We found no evidence, however, that production of IL-17 or IL-22 increased before or at onset of GVHD (Figure 1E-F).
Th17 cells are decreased in the skin of patients with aGVHD
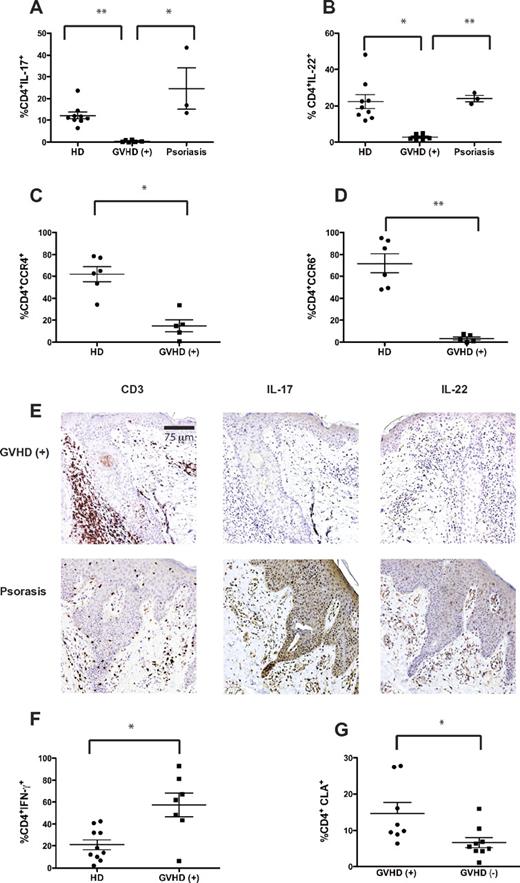
Th17 cells have a major role in the pathogenesis of human psoriasis,13 and 2 mouse studies suggest that Th17 cells are correlated with cutaneous aGVHD.8,14 We hypothesized that, despite the lack of circulating IL-17+ T cells, Th17 cells could preferentially accumulate within the target tissues of aGVHD. Skin biopsies were obtained from 7 patients with aGVHD before commencing prednisone, and skin-resident T cells isolated from cultured explants15 were analyzed for the production of IL-17, IL-22, and IFN-γ. Few IL-17+ or IL-22+ CD4+ T cells were present in the skin of patients with aGVHD, and the proportions were lower compared with T cells from biopsies of healthy or psoriatic skin (Figure 2A-B; supplemental Figure 2). To confirm the reduction in Th17 cells in skin affected by aGVHD, we measured expression of CCR4 and CCR6, characteristic homing markers of Th17 cells.16 In keeping with the lack of Th17 cells, the proportion of CD4+ T cells expressing CCR4 or CCR6 was significantly lower in skin from GVHD+ patients compared with that from healthy controls (Figure 2C-D).
Skin affected by cutaneous GVHD is enriched in Th1 but not Th17 cells. Punch biopsies from skin affected by aGVHD collected at the time of diagnosis (n = 7), psoriasis (n = 3), and healthy controls (n = 10) were cultured on 3-dimensional matrices in the presence of T-cell growth factors. After 3 weeks, suspension cells were restimulated with phorbol myristate acetate and ionomycin and gated CD4+ T cells were analyzed for the proportion of (A) IL-17 (GVHD+, 0.44% ± 0.18%; psoriasis, 24.6% ± 9.46%; healthy donors, 12.24% ± 1.60%), (B) IL-22 (GHVD+, 2.72% ± 0.61% psoriasis 24.05% ± 1.74%; healthy donors, 22.4% ± 3.82%), and (F) IFN-γ–producing cells (GVHD+, 57.36% ± 10.75%; healthy donors, 21.06% ± 4.63%). Unstimulated samples were analyzed for expression of (C) CCR4 (GVHD+, 14.88% ± 5.44%; healthy donors, 61.80% ± 6.73%) and (D) CCR6 (GVHD+, 3.4% ± 1.46%; healthy donors, 71.97% ± 8.87%). (G) Expression of cutaneous lymphocyte-associated antigen on circulating CD4+ T cells was measured in patients with cutaneous GVHD (14.69% ± 2.99%, n = 8) and compared with time-matched transplanted patients without GVHD (6.62% ± 1.43%, n = 9). (E) CD3 staining was used to identify T-cell infiltrates, and IL-17/IL-22 staining was used to identify cytokine expression patterns in the skin of patients with aGVHD (n = 6) and psoriasis (n = 1). The sections were examined using an Olympus BX61 microscope equipped with a 20×/0.75 objective lens and images were taken with an Olympus DP71 digital camera and acquired with DP controller 3.1.1.267 Olympus software. *P < .05. **P < .0001. Error bars represent SEM.
Skin affected by cutaneous GVHD is enriched in Th1 but not Th17 cells. Punch biopsies from skin affected by aGVHD collected at the time of diagnosis (n = 7), psoriasis (n = 3), and healthy controls (n = 10) were cultured on 3-dimensional matrices in the presence of T-cell growth factors. After 3 weeks, suspension cells were restimulated with phorbol myristate acetate and ionomycin and gated CD4+ T cells were analyzed for the proportion of (A) IL-17 (GVHD+, 0.44% ± 0.18%; psoriasis, 24.6% ± 9.46%; healthy donors, 12.24% ± 1.60%), (B) IL-22 (GHVD+, 2.72% ± 0.61% psoriasis 24.05% ± 1.74%; healthy donors, 22.4% ± 3.82%), and (F) IFN-γ–producing cells (GVHD+, 57.36% ± 10.75%; healthy donors, 21.06% ± 4.63%). Unstimulated samples were analyzed for expression of (C) CCR4 (GVHD+, 14.88% ± 5.44%; healthy donors, 61.80% ± 6.73%) and (D) CCR6 (GVHD+, 3.4% ± 1.46%; healthy donors, 71.97% ± 8.87%). (G) Expression of cutaneous lymphocyte-associated antigen on circulating CD4+ T cells was measured in patients with cutaneous GVHD (14.69% ± 2.99%, n = 8) and compared with time-matched transplanted patients without GVHD (6.62% ± 1.43%, n = 9). (E) CD3 staining was used to identify T-cell infiltrates, and IL-17/IL-22 staining was used to identify cytokine expression patterns in the skin of patients with aGVHD (n = 6) and psoriasis (n = 1). The sections were examined using an Olympus BX61 microscope equipped with a 20×/0.75 objective lens and images were taken with an Olympus DP71 digital camera and acquired with DP controller 3.1.1.267 Olympus software. *P < .05. **P < .0001. Error bars represent SEM.
To exclude the possibility that the paucity of IL-17– and IL-22–producing cells in GVHD+ skin was related to an artifact of in vitro culture, we quantitated the relative frequency of CD3+, IL-17+, and IL-22+ cells within skin biopsies using immunohistochemistry. These studies confirmed that, compared with controls, very few T cells from cutaneous GVHD lesions produce IL-17 or IL-22 (Figure 2E; supplemental Figure 3). These histologic data support the notion that Th17 cells are not responsible for the tissue damage in cutaneous GVHD.
In contrast to the paucity of Th17 cells, skin from patients with aGVHD (n = 7) contained a higher percentage of IFN-γ+ CD4+ T cells versus healthy donors (Figure 2F; supplemental Figure 4). These data are consistent with the fact that T cells infiltrating the skin of aGVHD patients express CCR5, a Th1-associated chemokine receptor.17 Notably, significantly more circulating CD4+ T cells expressed cutaneous lymphocyte-associated antigen in GVHD+ compared with GVHD− patients and healthy controls (Figure 2G), confirming that T cells in GVHD+ patients are poised to home to the skin.
Controversy exists in both mice and humans regarding the role of Th17 cells in the pathogenesis of aGVHD. In this prospective study of circulating and tissue-localized Th17 cells, we found no evidence that an increase in Th17 cells was associated with the onset of aGVHD. Rather, the lack of Th17 cells correlated with aGVHD, a finding consistent with data in mice showing that the absence of Th17 cells leads to augmented Th1 differentiation and exacerbation of aGVHD.5 Our data are consistent with histologic data documenting a low number of IL-17–producing cells in gut and skin in aGVHD patients,10 but contrary to a report that showed an increase in Th17 cells in the peripheral blood of patients with GVHD.9 The latter study found increased Th17 cells in the tissues of patients with chronic GVHD but did not evaluate patients with aGVHD. Compared with the latter study, our population was less heterogeneous (age, cell source, conditioning regimen, and GVHD prophylaxis). As our work focused on skin pathology, it remains possible that the cytokine profile of T cells present in other tissue sites, such as the gastrointestinal tract and liver, differs. Notably, in humans, granulocyte colony-stimulating factor mobilized PBMCs have reduced Th17 cells,18 and cyclosporine19,20 can inhibit IL-17 production. Thus, treatment regimen, stem cell source, and immunosuppression may all impact the cytokine profile of pathogenic cells. In conclusion, we found no evidence that an expansion of circulating or skin-localized Th17 cells was associated with onset of aGVHD. Because Th17 cells are clearly capable of causing skin inflammation in psoriasis,13 an outstanding question follows: what drives the local differentiation and/or homing of distinct CD4+ T-cell subsets in the skin and the subsequent development of inflammatory skin disorders? Knowledge that Th17 cells are not associated with the onset of aGVHD suggests that treatment options targeting this axis of T-cell development and effector function may have minimal therapeutic effect.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anna Koochin (Hematology Cell Bank) and Karen Lambie (Stem Cell Assay Laboratory, Terry Fox Laboratory) for collection of the patient samples, the Hematology Apheresis Unit at Vancouver General Hospital for providing PBMCs, Drs Bryce Cowan and Nicholas Carr (Department of Plastic Surgery, University of British Columbia) for providing healthy skin for normal controls, and Dr Youwen Zhou for providing samples from patients with psoriasis.
This work was supported by the Canadian Institute Health Research (MOP93793 and SAC-92849), the Hematology Research & Clinical Trials Unit, the Leukemia & Lymphoma Society of Canada, Vancouver Coastal Health Research Institute Venture Grant, and StemCell Technologies Inc. Core support for flow cytometry was funded by the MSFHR Immunity and Infection Research Center Unit. R.B. holds a University of British Columbia Academic Enhancement Award. J.D. is a Senior Scholar of the Michael Smith Foundation for Health Research and the Child and Family Research Institute. M.K.L. holds a Canada Research Chair in Transplantation.
Authorship
Contribution: R.B. designed the research, analyzed data, and wrote the paper; J.Y., V.C., C.K., and K.B. performed the experiments and analyzed data; M.G. performed immunohistochemistry analyses; M.M. contributed to immunohistochemistry analysis; J.D. contributed to immunohistochemistry analysis and participated in writing the manuscript; A.T. collected and summarized clinical data; and M.K.L. designed the research, analyzed data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raewyn Broady, Division of Haematology, University of British Columbia, 2775 Laurel St, Vancouver, BC, Canada V5Z 1M9; e-mail: rbroady@interchange.ubc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal