Abstract
The effects of Notch signaling on human megakaryocytic and erythroid differentiation were investigated by exposing human CD34+ progenitor cells to an immobilized chimeric form of the Notch ligand, Delta-like4 (Dll4Fc). Exposure of human cord blood CD34+ cells to Dll4Fc induced a modest enhancement of erythroid cell production. Conversely, under megakaryocytic culture conditions, Dll4Fc strongly impaired platelet production by reducing the generation of mature CD41a+CD42b+ megakaryocytes (MKs) and platelet-forming cells. The inhibitory activity of Dll4 on terminal MK differentiation was confirmed by culturing CD34+ cells onto Dll-4–expressing stroma cells (engineered to express the membrane-anchored form of Dll4). The reduced production of mature CD41a+CD42+ cells was rescued by inhibiting Notch signaling either with the N-N-(3,5-difluorophenacetyl-L-alanyl)-S-phenylglycine t-butyl ester γ-secretase inhibitor or the dominant-negative version of Mastermind. Dll4 impaired the generation of mature CD41a+CD42b+ cells and proplatelet formation without affecting earlier steps of MK differentiation, such as production of megakaryocytic/erythroid progenitors and colony-forming units–MKs. This blockade was accompanied by a modulation of the transcriptional program of megakaryocytic differentiation. All these results indicate that Dll4/Notch signaling inhibits human terminal MK differentiation.
Introduction
The Notch signaling pathway guides cell-fate decisions in multiple developmental processes.1,2 Intercellular communications that control the developmental fate of multipotent cells are mediated by the Notch family of transmembrane receptors in several invertebrate and vertebrate developmental systems. The Notch proteins are single-pass receptors that are activated by the Delta (or Delta-like) and Jagged/Serrate families of membrane-bound ligands.3 To date, 4 human Notch genes have been identified and all are expressed on hematopoietic cells.2 Also, 5 human Notch ligands, Delta-like1/3/4, and Jagged1/2 were identified, and all were shown to bind to Notch-1, Notch-2, and Notch-4.4,5 Notch receptors are matured in the secretory pathway and presented at the cell surface as heterodimeric molecules. Interaction with ligands leads to 2 additional proteolytic cleavages that liberate the Notch intracellular domain (NICD) from the plasma membrane. NICD enters the nucleus, where it interacts with the DNA-binding protein, CSL (C-promoter binding factor [CBF]-1, Suppressor of Hairless, LAG-1; also known as recombination signal-binding protein Jk [RBP-Jk]).6 In the absence of NICD, CSL represses transcription through interactions with a corepressor complex, containing a histone deacetylase. Upon entering the nucleus, NICD displaces the corepressor complex from CSL and replaces it with a transcriptional activation complex that includes NICD, Mastermind (MAML-1), the histone acetyltransferase p300, and, possibly, PCAF p300/CBP (cyclic AMP response element-binding protein [CREB]-binding protein)–associated factor. Notch signaling, thus, converts CSL from a repressor to an activator, leading to the transcription of target genes. The target genes include members of the Hes and HRT/HERP/Hey families of transcriptional repressors; therefore, Notch signaling is often viewed as a transcription cascade.
The classical view holds that Notch signaling controls the balance between the progenitor pool and its differentiating progeny and thus is involved in the maintenance of stem cell fate.1,2 In fact, a number of studies have provided evidence that ligand-induced Notch signaling favored hematopoietic stem cell (HSC) self-renewal, increased the numbers of progenitors, and promoted HSC survival. Moreover, Notch signaling may be instructive for differentiation toward a particular fate. It plays a crucial role in the hematopoietic system, especially in the regulation of the T-cell lymphoid lineage commitment7-9 and in late stages of B-cell development.10 Because of the key role of Notch signaling in supporting early T-cell differentiation, it was generally established that Notch concomitantly negatively regulates myeloid lineage development. The megakaryocytic and erythroid lineages are extremely linked, because they share a common bipotent progenitor called the MEP (MK/erythroid progenitor). The role of Notch in megakaryocytic and erythroid development remains a matter of debate. While some data report that Notch signaling represses human megakaryocytic and/or erythroid differentiation,11-13 other reports indicate that they act instead as inductors of erythroid or megakaryocytic differentiation.14-16
To further gain insights into the implication of Notch signaling in human megakaryocytopoiesis and erythropoiesis, we investigated the capacity of human progenitor CD34+ cells to produce megakaryocytes (MKs) and platelets as well as erythrocytes in culture onto the membrane-anchored form of the Notch ligand Delta-like4 (Dll4) or an immobilized Dll4-Fc chimeric molecule. Dll-4, which is expressed in a wide range of adult and fetal tissues, including major sites of hematopoiesis, such as the thymus, bone marrow, lymph nodes, and fetal liver,17,18 plays a key role in T-cell differentiation7,8 as well as vasculogenesis.19 We previously observed that Delta4-expressing stromas reduced in vitro HSC proliferation and favored HSC self-renewal by mechanisms independent of the mitotic history.20,21 Our present results showed that the Notch/Dll4 signaling strongly impairs human terminal megakaryocytic differentiation, with a limited promotion toward erythroid differentiation.
Methods
Cell preparation and labeling
Human CD34+ cells were obtained, after informed consent in agreement with our Institute Ethic Committee (Assistance Publique des Hôpitaux de Paris) and in accordance with the Declaration of Helsinki, either from leukapheresis samples after mobilization performed on patients or from umbilical cord blood. CD34+ cells were isolated by a positive selection using an immunomagnetic cell-sorting system (AutoMacs; Miltenyi Biotec).
Flow cytometric analysis and cell sorting
All antibodies were obtained from BD Pharmingen (BD Bioscience) or Biolegend (eBioscience). For purification of murine Lineage-, Sca-1+, and ckit+ (LSK) cells by flow cytometry, murine bone marrow cells were first magnetically depleted of lineage-positive cells using a cocktail of anti–mouse antibodies against all mature blood cells, including Ter119, B220 CD4, CD5, CD11b, and Gr-1, followed by incubation with magnetic bead-coupled sheep anti–rat antibody (Dynal; Invitrogen). Residual lineage-positive cells were detected using a goat-anti–rat phycoerythrin (PE)–conjugated antibody (Clinisciences), and LSK cells were sorted using FACSDiva after a subsequent staining with anti–c-kit–APC and Sca-1–fluorescein isothiocyanate (FITC).
Human megakaryocytic differentiation was followed through the analysis of CD41a and CD42b expression. The ploidy of CD41a+ cells was examined by labeling with Hoeschst 33 342. Human erythroid differentiation was followed through with analysis of CD36 and glycophorin A (GPA) expression. Murine MKs were identified as CD41a+ cells, and murine myeloid cells were identified as Mac-1+ cells.
Culture on Dll4Fc-coated plates for erythroid differentiation
For erythroid differentiation, human cord blood CD34+ cells were cultured in serum-free medium with 10% fetal calf serum (FCS; Hyclone) in the presence of human erythropoietin (huEPO; 1 U/mL; Kirin Brewery), human interleukin-3 (huIL-3; 50 U/mL) and human stem-cell factor (huSCF; 50 ng/mL; Kirin) in 96-well plates coated with Dll4Fc, as previously described.20 Coated human immunoglobulin G1 (IgG1) was used as control. Ingredients used to prepare the serum-free medium were Iscove modified Dulbecco medium supplemented with 1.5% bovine serum albumin (BSA; Cohn fraction V; Sigma-Aldrich), sonicated lipids, and Fe3+-saturated human transferring. Seven days later, cells were seeded onto a new plated coated with Dll4Fc on the same culture conditions with 30% FCS. Cells were analyzed throughout the culture for the expression of CD36 and GPA by flow cytometry.
Culture on Dll4Fc-coated plates for MK differentiation
Human cord blood or mobilized peripheral blood CD34+ cells were cultured in serum-free medium in the presence of recombinant human thrombopoietin (huTPO; 10 ng/mL; Kirin Brewery) and recombinant huSCF (25 ng/mL; Amgen) in 96-well plates coated with Dll4Fc. Ingredients used to prepare the serum-free medium were included as previously described.22 Coated human IgG1 was used as a control. Five days later, cells were seeded onto a new plated coated with Dll4Fc in the same culture conditions. Then, 5-6 days later, cells were harvested, counted, CD41a+ cells were examined for their ploidy, and CD41a+CD42b+ cells were sorted (FacsDIVA; BD Biosciences) to be seeded onto a new Dll4Fc-coated plate in serum-free medium containing huTPO (10 ng/mL). Proplatelet-forming cells were scored 3-4 days later under an inverted microscope (Zeiss).
Some cultures were performed in the presence of DAPT (a γ-secretase inhibitor: N-3,5-difluorophenacetyl)-L-alanyl-S-phenylglycine-t-bytyl ester; Calbiochem) at a final concentration of 10μM or dimethyl sulfoxide (DMSO) as a control.
Some cultures were initiated with another cocktail of cytokines: recombinant huIL-3 (100 IU/mL), recombinant huIL-6 (10 ng/mL), recombinant huSCF (25 ng/mL), and recombinant human fetal liver tyrosine kinase 3 ligand (FLT-3L; 10 ng/mL).
Viral infection
MIGR(DN-MAML1) and MIGR constructs (kind gift from Warren Pear, University of Pennsylvania, Philadelphia, PA) are described elsewhere.23 Viral supernatants were obtained as previously described.24 For transduction, 105 human cord blood CD34+ cells were incubated with the viral supernatants in serum-free medium containing huIL-3 (100 U/mL), huTPO (50 ng/mL), huSCF (50 ng/mL), and huFLT3-L (100 mg/mL) for 48 hours to be thereafter extensively washed and cultured for 8-10 days in Dll4Fc-coated plates in serum-free medium in the presence of huTPO (10 ng/mL) and huSCF (25 ng/mL) before flow cytometric analyses.
Coculture on stromal cells for MK differentiation
OP9-GFP, OP9-HuD1, OP9-HuD4, OP9-MuD1, and OP9-MuD4 stromal cells were maintained in “OP9-media:” α-modified Eagle medium (MEM; Invitrogen) supplemented with 20% FCS (Hyclone), 2mM glutamine (Gibco), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Invitrogen).25 The stromal cells were plated 1 day before the start of coculture in 24-well plates at a density of 5 × 104 cells/well. Human CD34+ cells were plated onto the stromal cells in serum-free medium containing 5% FCS, huTPO (10 ng/mL), and huSCF (25 ng/mL), and cells were seeded onto fresh OP9 cells 5 days later before flow cytometric analysis. Some cultures were performed in cytokine-free medium.
Next, 10 000 LSK cells were cultured onto the stromal cells (OP9-GFP, OP9-MuD1, and OP9-MuD4) in OP9 culture medium containing 5% FCS, huTPO (10 ng/mL), and muSCF (25 ng/mL) for 7 days before flow cytometric analysis. Some cultures were performed in cytokine-free medium.
Clonogenic assays
RNA extraction and real-time RT-PCR
RNA was isolated using TriZol (Invitrogen). The expression levels of globin transcription factor 1 (GATA-1), Hes-1, erythroid Krüppel-like factor (EKLF), acute myeloid leukemia-1 (AML-1), Friend leukemia integration-1 (FLI-1), c-myb, nuclear factor (NF)–E2, tubulin β1, Notch1, Notch2, and Notch4 were assessed by quantitative reverse-transcription polymerase chain reaction (RT-PCR). Expression levels of GATA-1, Hes-1, AML-1, FLI-1, c-myb, NF-E2 Tubulin-β1, Notch1, Notch2, and Notch4 were normalized to hypoxanthine phosphoribosyltransferase (HPRT; sequences are described in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Probes were labeled at the 5′-end with cefamandole (FAM) and at the 3′-end with tetramethylrhodamine (TAMRA; Eurogentec). PCR amplification was carried out for 10 minutes at 95°C, followed by 50 cycles (1 minute at 60°C and 15 seconds at 95°C) in ABI 7500 Real (Applied Biosystems). The mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene:HPRT ratio, with endogenous standard in human erythroleukemia (HEL) cells (ATCC) as a positive standard (ie, normalized target value).
Statistical analysis
Statistical significance of differences between the different conditions was assessed using a 2-tailed t test.
Results
The Dll4 ligand inhibits terminal MK differentiation of human CD34+ cells in vitro
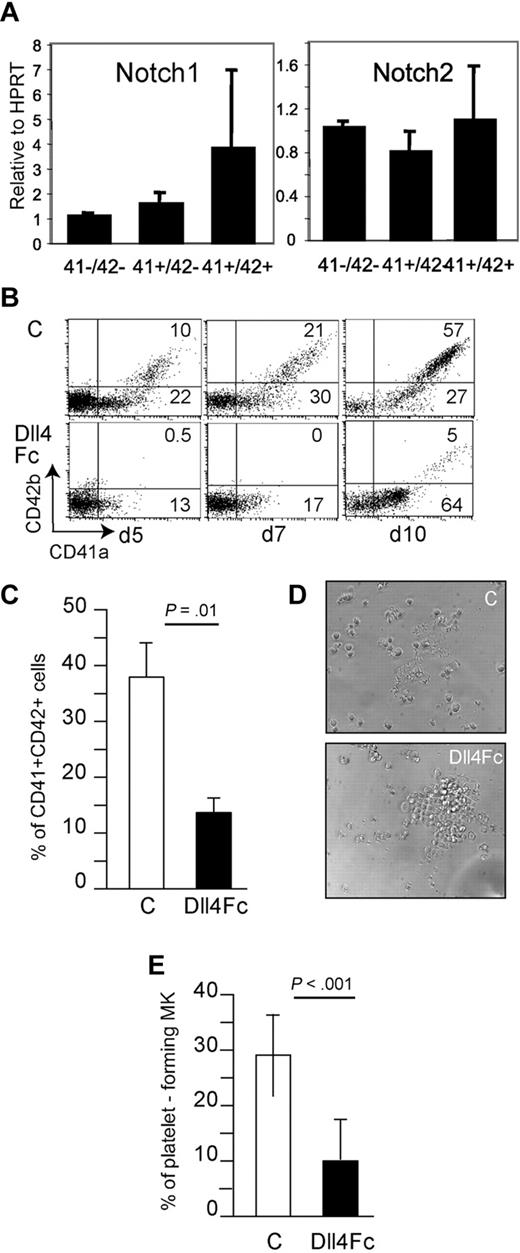
As a first approach to investigate the role of Notch signaling in the regulation of megakaryocytic development, Notch1, Notch2, and Notch4 expressions were examined in different phenotypic cell populations: CD41a−CD42b− cells and immature CD41a+CD42b− and mature CD41a+CD42b+ MK populations. Human cord blood CD34+ cells were cultivated under megakaryocytic culture conditions (huTPO and huSCF), and Notch expressions were analyzed 5 days after initiation of the culture. We found that Notch1 and Notch2 transcripts were expressed in the 3 tested cell populations (Figure 1A). By contrast, Notch4 was only barely detected in differentiated MK (CD41a+CD42b+ cells, data not shown). We reasoned that the maintenance of Notch1/2 receptor expression along megakaryocytic differentiation might imply a role of the Notch signaling pathway in human MK differentiation. To test this hypothesis, megakaryocytopoiesis was analyzed by culturing human cord blood CD34+ cells under megakaryocytic culture conditions in the presence or absence of immobilized Dll4Fc. The use of such a chimeric Notch ligand, which mimics the membrane-bound form,20 instead of Dll4-expressing stromas, allows us to examine terminal MK differentiation, such as platelet-forming cells. When the cultures were initiated with 15 000 input CD34+ cells, the absolute number of output nucleated cells at day 10 was 1.7 ± 0.7 × 106 cells for control cultures versus 0.9 ± 0.5 × 106 for Dll4Fc-treated cultures. This decreased cell expansion was previously observed when human CD34+ cells were exposed to membrane-bound Dll4.20 Cytometric analyses performed along the cultures (5, 7, and 10 days after the initiation of the cultures) showed a reduction in the proportion of immature CD41a+CD42b− in cell populations exposed to Dll4Fc. More obvious, the proportion of mature CD41a+CD42b+ cells, which increased upon TPO stimulation in the absence of Dll4Fc, did not display such an important increase in the presence of Dll4Fc. This difference was evidenced as soon as day 5 of culture (Figure 1B) and was maintained throughout the culture. At day 10, we found a significant decrease in the proportion of CD41a+CD42b+ cells (Figure 1C), leading to a diminution of the absolute number of output mature MK (6.6 ± 1.0 × 105 for control culture versus 1.2 ± 0.3 × 105 cells for Dll4Fc-treated culture; P < .01; n = 6). A similar reduction in mature MK was obtained with adult CD34+ cells derived from mobilized peripheral blood (supplemental Figure 1B-C). Furthermore, such Dll4Fc-treated culture displayed few polyploid MK (≥ 8N), compared with control cultures (2% ± 0.5% for Dll4Fc-treated culture versus 16% ± 2% for control cultures (supplemental Figure 1A,D,E). A 4-day extension of the culture did not increase the proportion of CD41a+CD42b+cells in Dll4Fc-treated populations (data not shown). Importantly, when CD41a+CD42b+ cells were sorted after 10 days of culture and plated 3 more days in the presence of huTPO with or without Dll4Fc, a decrease in the proportion of proplatelet-forming cells was observed by phase-contrast microscopy (29% ± 9% of CD41a+CD42b+ cells from control culture showed proplatelet protrusions while only 10% ± 8% of CD41a+CD42b+ cells from Dll4Fc culture showed these cellular modifications; Figure 1C-D).
Dll4 represses terminal MK differentiation in vitro. Human cord blood CD34+ cells were plated in the presence of huTPO and huSCF. (A) Notch1 and 2 expression throughout megakaryocytic differentiation. Analysis by quantitative RT-PCR of the expression levels of Notch1 and Notch2 in sorted cell populations at day 5 throughout MK differentiation. mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene/HPRT ratio in HEL cells. Results were obtained from 3 independent experiments. Sequences of the PCR primers are described in supplemental Table 1. Human cord-blood CD34+ cells were plated onto control or Dll4Fc-coated wells in the presence of huTPO and huSCF. (B-C) Flow cytometric analysis of MK differentiation from human cord blood CD34+ cells plated onto control or Dll4Fc-coated wells in the presence of huTPO and huSCF. Flow cytometric analyses were performed at days 5, 7, and 10 (1 representative experiment). (C) Histogram representation of the % of CD41a+CD42b+ cells at day 10 of the culture. Mean ± SEM from 6 independent experiments. (D-E) At day 10, sorted CD41a+CD42b+ cells were plated onto Dll4Fc-coated wells in the presence of huTPO, and proplatelet-forming cells were evaluated 3-4 days later. (D) Phase-contrast microscopy of days 13-14 in control and Dll4Fc cultures. Original magnification was ×100. (E) Histogram representation of phase contrast microscopy analyses of platelet-forming cells are presented in (E). Mean ± SEM from 7 independent experiments.
Dll4 represses terminal MK differentiation in vitro. Human cord blood CD34+ cells were plated in the presence of huTPO and huSCF. (A) Notch1 and 2 expression throughout megakaryocytic differentiation. Analysis by quantitative RT-PCR of the expression levels of Notch1 and Notch2 in sorted cell populations at day 5 throughout MK differentiation. mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene/HPRT ratio in HEL cells. Results were obtained from 3 independent experiments. Sequences of the PCR primers are described in supplemental Table 1. Human cord-blood CD34+ cells were plated onto control or Dll4Fc-coated wells in the presence of huTPO and huSCF. (B-C) Flow cytometric analysis of MK differentiation from human cord blood CD34+ cells plated onto control or Dll4Fc-coated wells in the presence of huTPO and huSCF. Flow cytometric analyses were performed at days 5, 7, and 10 (1 representative experiment). (C) Histogram representation of the % of CD41a+CD42b+ cells at day 10 of the culture. Mean ± SEM from 6 independent experiments. (D-E) At day 10, sorted CD41a+CD42b+ cells were plated onto Dll4Fc-coated wells in the presence of huTPO, and proplatelet-forming cells were evaluated 3-4 days later. (D) Phase-contrast microscopy of days 13-14 in control and Dll4Fc cultures. Original magnification was ×100. (E) Histogram representation of phase contrast microscopy analyses of platelet-forming cells are presented in (E). Mean ± SEM from 7 independent experiments.
Accordingly, such an inhibition of mature MK production was also observed after exposure of CD34+ cells to OP9 stroma engineered to express the physiological membrane-bound human Dll4 (HuDll4)–green fluorescent protein(GFP). Notably, the reduction was more important than with the chimeric Dll4Fc ligand, from 19% ± 5% CD41a+CD42b+ in the presence of OP9-GFP to 1.5% ± 0.5% in the presence of OP9-HuDll4 (Figure 2), Interestingly, CD34+ cells cultured onto OP9-HuDll1 also gave rise to very few CD41a+CD42b+ cells (1.5% ± 0.5% in the presence of OP9-HuDll4 vs 1% ± 0.5% in the presence of OP9-HuDll1; Figure 2), indicating that Dll1 has the same inhibitory effect on human megakaryocytic differentiation as Dll4.
Membrane-bound Dll4 and Dll1 repress MK differentiation from CD34+ cells. (A) Human cord blood CD34+ cells were cultured for 10 days onto control OP9-GFP or OP9-huDll1 or OP9-huDll4 stroma in the presence of huSCF and huTPO before flow cytometric analyses (1 representative experiment). (D) Histogram representation of the % of CD41a+CD42b+ cells at day 10 of the culture. Mean ± SEM from 3 independent experiments.
Membrane-bound Dll4 and Dll1 repress MK differentiation from CD34+ cells. (A) Human cord blood CD34+ cells were cultured for 10 days onto control OP9-GFP or OP9-huDll1 or OP9-huDll4 stroma in the presence of huSCF and huTPO before flow cytometric analyses (1 representative experiment). (D) Histogram representation of the % of CD41a+CD42b+ cells at day 10 of the culture. Mean ± SEM from 3 independent experiments.
To assess whether Dll4 might inhibit TPO signaling and, subsequently, megakaryocytic differentiation, human cord-blood CD34+ cells were plated in the presence of IL-3/IL-6/SCF/FLT3-L for 11 days. In such a cytokinic context, cytometric analysis (supplemental Figure 2) revealed that MK differentiation was also inhibited, indicating that Dll4 MK inhibition did not specifically depend on TPO signaling.
Altogether, these results obtained with different approaches strongly suggest that in vitro exposure of human cord blood and mobilized CD34+ cells to Dll4 reduces terminal MK differentiation and proplatelet formation, and point out the fact that Notch signaling may play an important role in limiting cytokine-induced human platelet production.
The Dll4/Notch ligand slightly promotes erythroid differentiation in vitro
The role of the Dll4/Notch pathway on erythroid differentiation was also investigated by cultivating human cord blood CD34+ cells under erythroid culture conditions (HuSCF, Hu IL-3, and HuEPO) for 17 days in plates precoated with immobilized Dll4Fc. Cytometric analysis of CD36 and GPA expression performed throughout the culture revealed a limited increase in the proportion of erythroid cells, as illustrated by a slightly higher proportion of CD36+GPA− at days 5, 10, and 17 (P < .05; n = 3) and of CD36+GPA+ cells at days 10 and 17 in cells exposed to Dll4Fc (P < .05; n = 3; Figure 3A). Interestingly, Notch-1 and -2 expressions were down-regulated during erythroid differentiation (from CD36-GPA- cells to CD36+GPA+ cells; Figure 3B). These data showed that Notch/Dll4 exerted a limited promotion of erythroid differentiation, which was associated with a decreased expression of Notch during erythroid differentiation.
Dll4Fc slightly promotes erythroid differentiation. (A) Kinetic analysis of erythroid differentiation from human cord blood CD34+ cells cultured onto control or Dll4Fc-coated wells in the presence of Hu EPO, Hu SCF, and HuIL-3. Flow cytometric analyses were performed at days 5, 7, 10, 12, 14, and 17. Histogram representation of cytometric analysis for CD36 and GPA markers (mean ± SEM from 3 independent experiments). (B) Notch1 and 2 expression is downmodulated throughout erythroid differentiation. Analysis by quantitative RT-PCR of the expression levels of Notch1 and Notch2 in sorted cell populations at day 11 throughout erythroid differentiation. mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene:HPRT ratio in HEL cells. Results were obtained from 3 independent experiments.
Dll4Fc slightly promotes erythroid differentiation. (A) Kinetic analysis of erythroid differentiation from human cord blood CD34+ cells cultured onto control or Dll4Fc-coated wells in the presence of Hu EPO, Hu SCF, and HuIL-3. Flow cytometric analyses were performed at days 5, 7, 10, 12, 14, and 17. Histogram representation of cytometric analysis for CD36 and GPA markers (mean ± SEM from 3 independent experiments). (B) Notch1 and 2 expression is downmodulated throughout erythroid differentiation. Analysis by quantitative RT-PCR of the expression levels of Notch1 and Notch2 in sorted cell populations at day 11 throughout erythroid differentiation. mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene:HPRT ratio in HEL cells. Results were obtained from 3 independent experiments.
Canonical Notch signaling mediates Dll4-inhibition of human MK differentiation
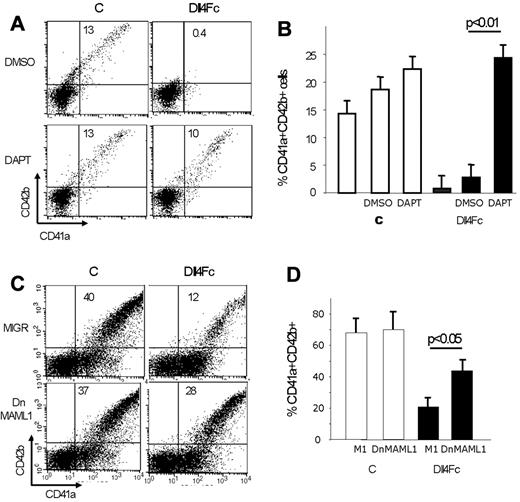
To ascertain that the inhibition of MK differentiation by Dll4 relied on Notch signaling, we inhibited this pathway using 2 strategies: (1) a gamma-secretase inhibitor (DAPT) that prevents the cleavage of the Notch receptors and (2) a dominant negative version of MAML1 (dnMAML1), an essential component of the Notch protein complex that entraps all 4 intracellular Notch (ICN1-4) in transcriptionally inactive CSL/ICN/dnMAML1 complexes. The inhibition of MK differentiation was almost completely abrogated when Dll4Fc-exposed cells were cultured in the presence of DAPT (Figure 4A-B; 24% ± 4% of CD41a+CD42b+ cells for Dll4Fc+DAPT versus 3% ± 1% of CD41a+CD42b+ cells for Dll4Fc+DMSO; n = 4). These findings show that the pharmacological agent, DAPT, inhibiting the Notch pathway restored MK differentiation. As a second strategy, transduction of human CD34+ cells with the dnMAML1-expressing MIGR retrovirus (Figure 4C) before initiation of culture onto Dll4Fc-coated plates rescued MK differentiation (28% of CD41a+CD42b+ cells for Dll4Fc+MIGRDnMAML1 vs 12% of CD41a+CD42b+ cells for Dll4Fc+emptyMIGR). We conclude that activation of the Notch signaling pathways was responsible for the inhibition of late MK differentiation induced by exposure of human CD34+ cells to Dll4.
Canonical Notch signaling mediates Dll4-inhibition of MK differentiation. (A-B) Human cord blood CD34+ cells were plated onto Dll4Fc-coated plates in the presence of 10μM DAPT or mock (DMSO) control. FACS analyses for CD41a and CD42b markers of cultured cells were performed after 10 days of cultures. (A) FACS analyses for CD41a and CD42b markers of 1 representative experiment. (B) Histogram representation of flow cytometric results presented in panel A. Mean ± SEM from 3 independent flow cytometric analyses after 10 days of culture is shown. (C) Human cord blood CD34+ cells were transduced with retrovirus encoding either DN-MAML1 or MIG-empty vector (the transduction rate was close to 30% for both retroviral vectors), and 2 days later, plated onto control and Dll4Fc-coated plates. FACS analyses of cultured cells were performed after 7 days of cultures onto Dll4Fc. Flow cytometric analysis of CD41a and CD42b markers in GFP+ cells from control and Dll4Fc cultures (1 representative experiment). (D) Histogram representation of flow cytometric results presented in panel C. Mean ± SEM from 5 independent flow cytometric analyses after 10 days of culture.
Canonical Notch signaling mediates Dll4-inhibition of MK differentiation. (A-B) Human cord blood CD34+ cells were plated onto Dll4Fc-coated plates in the presence of 10μM DAPT or mock (DMSO) control. FACS analyses for CD41a and CD42b markers of cultured cells were performed after 10 days of cultures. (A) FACS analyses for CD41a and CD42b markers of 1 representative experiment. (B) Histogram representation of flow cytometric results presented in panel A. Mean ± SEM from 3 independent flow cytometric analyses after 10 days of culture is shown. (C) Human cord blood CD34+ cells were transduced with retrovirus encoding either DN-MAML1 or MIG-empty vector (the transduction rate was close to 30% for both retroviral vectors), and 2 days later, plated onto control and Dll4Fc-coated plates. FACS analyses of cultured cells were performed after 7 days of cultures onto Dll4Fc. Flow cytometric analysis of CD41a and CD42b markers in GFP+ cells from control and Dll4Fc cultures (1 representative experiment). (D) Histogram representation of flow cytometric results presented in panel C. Mean ± SEM from 5 independent flow cytometric analyses after 10 days of culture.
Inhibition of MK differentiation is effective with exposure to Dll4Fc during the first 5 days of culture
Having shown that activating Notch-signaling pathways inhibits terminal megakaryocytic differentiation, we wanted to determine when its activation by Dll4Fc was required during the culture. To answer this question, we split the cultures into 3 periods, the 1-5-day, 5-10-day, and the 10-14-day periods. During each period, the cells were cultured either with or without Dll4Fc, and MK differentiation was assessed. Cytometric analysis of CD41a and CD42b expression showed that the presence of Dll4Fc only during the first 5 days was sufficient to induce an inhibition of MK differentiation (Figure 5A), which is in agreement with the reduced proportion of CD41a+CD42b+ cells previously evidenced as soon as day 5 of culture (Figure 1B). In contrast, the presence of Dll4Fc only during the second part of the culture (the 5-10-day period) did not significantly impair the generation of mature CD41a+CD42b+ cells. As a second parameter to follow MK differentiation, we measured the generation of proplatelet-forming cells at day 14, after having sorted mature CD41a+CD42b+ cells at day 10. When cells were exposed to Dll4Fc only during a short 5-day period, an inhibition in the formation of platelet-forming MK was evidenced (Figure 5B), which is in agreement with the reduced production of CD41a+CD42b+ cells. These data indicated that the presence of Dll4Fc only during the first days of the culture was sufficient to inhibit terminal MK differentiation (generation of CD41a+CD42b+ cells and proplatelet-forming cells).
The presence of Dll4Fc during the 5 first days of cultures is sufficient to inhibit terminal MK differentiation. Human cord blood CD34+ cells were plated onto control or Dll4Fc-coated wells in the presence of huTPO and huSCF. At day 5, cells were plated onto freshly prepared control or Dll4Fc-coated wells. At day 10, sorted CD41a+CD42b+ cells were plated onto control or Dll4Fc-coated wells in the presence of huTPO, and the % of platelet-forming cells was evaluated 4 days later. (A) Histogram representation of the % of CD41a+CD42b+ cells after 10 days of culture. Mean ± SEM from 3 independent experiments. Black bars represent culture period performed in the presence of Dll4Fc, while white bars represent culture period without Dll4Fc. (B) Histogram representation of phase-contrast microscopy analyses of the % of platelet-forming cells among sorted CD41a+CD42b+ cells (mean ± SEM from 3 independent experiments).
The presence of Dll4Fc during the 5 first days of cultures is sufficient to inhibit terminal MK differentiation. Human cord blood CD34+ cells were plated onto control or Dll4Fc-coated wells in the presence of huTPO and huSCF. At day 5, cells were plated onto freshly prepared control or Dll4Fc-coated wells. At day 10, sorted CD41a+CD42b+ cells were plated onto control or Dll4Fc-coated wells in the presence of huTPO, and the % of platelet-forming cells was evaluated 4 days later. (A) Histogram representation of the % of CD41a+CD42b+ cells after 10 days of culture. Mean ± SEM from 3 independent experiments. Black bars represent culture period performed in the presence of Dll4Fc, while white bars represent culture period without Dll4Fc. (B) Histogram representation of phase-contrast microscopy analyses of the % of platelet-forming cells among sorted CD41a+CD42b+ cells (mean ± SEM from 3 independent experiments).
The Dll4/Notch ligand does not inhibit early steps of human MK differentiation in vitro
To characterize the stage of MK differentiation at which Dll4/Notch signaling exerts its inhibitory role, we assessed the effects of Dll4Fc exposure at different stages of differentiation. After 5 days of culture, the % of the MK/erythroid cell fraction, identified as CD34+CD38+CD45RA−CD123− cells, was strictly identical in both populations (19% ± 4% for control and Dll4 cultures; data not shown). Similarly, the % of CD34+ cells was barely decreased in cell populations exposed to Dll4, compared with control cul-tures (Figure 6A-B; not significant). The number of CFU-MK per 1500 output mononuclear cells was identical in both populations (Figure 6C). Interestingly, a weak immunocytochemical CD41 was observed on MK derived from the CFU-MK exposed to Dll4Fc (in correlation with the weaker CD41a expression evidenced, eg, in Figure 1B; data not shown). Altogether, these data indicate that Dll4Fc does not maintain a progenitor cell population. To assess whether Dll4 affected differentiation toward another lineage, we examined the CFU–granulocyte macrophage, burst-forming unit erythroid, and CFU-MK potential of the control and the Dll4-treated cultures at days 2 and 5 of the culture. We did not notice any difference in the number of burst-forming unit erythroid and CFU-GM between both conditions, indicating the absence of shift toward the erythroid or the myeloid lineage in our MK culture conditions (data not shown).
Dll4 does not inhibit early steps of MK differentiation. Human cord blood CD34+ cells were plated onto Dll4Fc-coated plates for 5 days. (A) FACS analyses for CD34 and CD41a markers of 1 representative experiment. (B) Histogram representation of the % of CD34+ cells at day 5 of the culture. Mean ± SEM from 9 independent experiments. (C) Total nucleated cells were plated on plasmatic coagulum for CFU-MK determination. Histogram representation of the number of CFU-MK in 1500 mononuclear cells (mean ± SEM from 4 independent experiments).
Dll4 does not inhibit early steps of MK differentiation. Human cord blood CD34+ cells were plated onto Dll4Fc-coated plates for 5 days. (A) FACS analyses for CD34 and CD41a markers of 1 representative experiment. (B) Histogram representation of the % of CD34+ cells at day 5 of the culture. Mean ± SEM from 9 independent experiments. (C) Total nucleated cells were plated on plasmatic coagulum for CFU-MK determination. Histogram representation of the number of CFU-MK in 1500 mononuclear cells (mean ± SEM from 4 independent experiments).
Dll4/Notch signaling repressed a MK transcriptional response
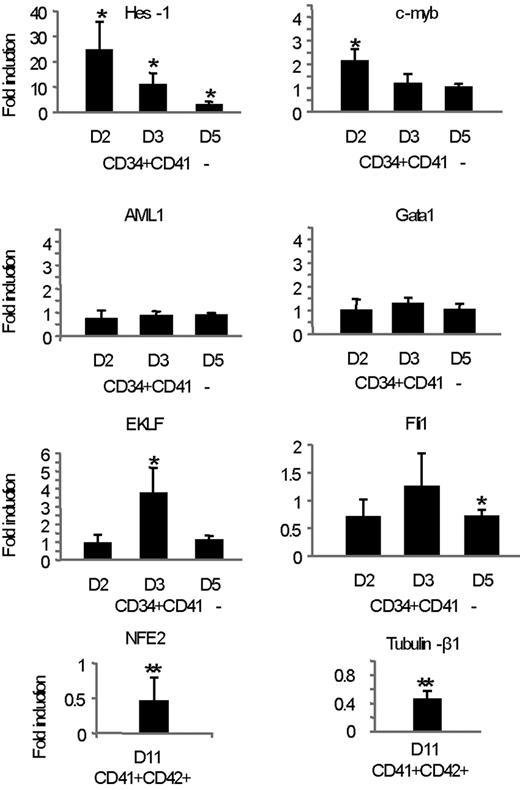
To gain insight into the molecular mechanisms involved in the inhibition of megakaryocytic differentiation by Notch/Dll4, we evaluated the expression level of several transcriptional factors involved in the regulation of megakaryocytic differentiation. We thus compared, by quantitative RT-PCR analysis, the expression level of these genes in control and Dll4-treated populations 2, 3, and 5 days after initiation of the cultures (Figure 7). This analysis was performed on purified CD34+CD41− cell populations, which represent more than 90% of the total population at days 2 and 3 in control and Dll4-treated cultures. Exposure to Dll4Fc increased the expression of the Notch target gene Hes-1. Indeed, Hes-1 expression was higher than in control at each tested time of the culture (x24 at D2, x11 at D3, and x3 at day 5). However, Dll4 impact on Hes-1 expression decreased with MK differentiation. The increase of Hes-1 expression was abrogated in the presence of DAPT (data not shown). AML-1 and GATA-1 expression (2 master genes modulated early during megakaryocytic differentiation28 ) was similar in both cultures conditions. Expression of c-myb and EKLF, 2 transcription factors known to inhibit megakaryocytopoiesis,28 were slightly enhanced in cells exposed to Dll4, compared with control cultures (at day 2 for c-myb x2.1 ± 0.3 P < .05, and at day 3 for EKLF, x3.1 ± 0.5; P < .01). Conversely, expression of FLI-1, a transcription factor involved in MK differentiation, was decreased at day 5 (x0.7 ± 0.1; P < .05) in CD34+CD41− cells exposed to Dll4, compared with control cultures. Interestingly, expression of NF-E2 and tubulin-β1 and 2 genes involved in platelet formation29,30 were decreased in sorted CD41+CD42+ cells exposed to Dll4, compared with control cultures. These findings indicate that Notch/Dll4 signaling modulates the expression of genes belonging to the transcriptional program of late MK differentiation.
Dll4/Notch inhibits a MK transcriptional program in human CD34+CD41− cells. Analysis by quantitative RT-PCR of the expression levels of various genes in sorted CD34+CD41− cells cultured onto control or Dll4Fc-coated plated harvested at days 2, 3, and 5 and in sorted CD41+CD42+ cells cultured onto control or Dll4-Fc-coated, plated, and harvested at day 11. mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene:HPRT ratio in HEL cells. For each gene, the relative expression levels in populations grown onto Dll4Fc are expressed as fold induction, compared with the levels (set to 1) detected in populations grown onto control plates. Results were obtained from 4 independent experiments. Sequences of the PCR primers are described in supplemental Table 1.
Dll4/Notch inhibits a MK transcriptional program in human CD34+CD41− cells. Analysis by quantitative RT-PCR of the expression levels of various genes in sorted CD34+CD41− cells cultured onto control or Dll4Fc-coated plated harvested at days 2, 3, and 5 and in sorted CD41+CD42+ cells cultured onto control or Dll4-Fc-coated, plated, and harvested at day 11. mRNA expression of each gene was normalized to that of HPRT mRNA and calibrated to the gene:HPRT ratio in HEL cells. For each gene, the relative expression levels in populations grown onto Dll4Fc are expressed as fold induction, compared with the levels (set to 1) detected in populations grown onto control plates. Results were obtained from 4 independent experiments. Sequences of the PCR primers are described in supplemental Table 1.
Dll4/Noch signaling favors murine early MK development
The presently described lack of Dll4 effect on early MK differentiation in human cells is at odds with those demonstrated recently in mice, describing Notch signaling as specifying MK development from hematopoietic stem cells.16,31 Indeed, the investigators observed the development of CD41+ cells when using heterotypic cocultures of murine Lin-Sca-1+cKit+ (LSK) cells with OP9 cells expressing Dll1, but not with parental OP9 cells.16 To verify the apparent discrepancy between human and murine cells, we have cultivated murine LSK cells onto OP-9MuDll4 (engineered to express murine membrane-bound Dll4), OP-9MuDll1 (engineered to express murine membrane-bound Dll1), or the control OP-9-GFP for 7 days (with or without cytokines). As previously reported,16 an enhancement in the proportion of CD41a+ cells in culture exposed to muDll4 and muDll1, was observed (supplemental Figure 3). These data unveil a paradox between humans and mice concerning the role of Notch in early megakaryocytopoiesis.
Discussion
One of the main conclusions of this study is that Notch/Dll4 signaling inhibits in vitro late stages of MK differentiation of human CD34+ cells. This conclusion is supported by observations showing that exposure of human cord blood or adult peripheral blood CD34+ cells to the chimeric Dll4Fc protein impaired platelet production by reducing the generation of mature CD41a+CD42b+ MK, and platelet-forming cells. The inhibitory role of Dll4 on terminal MK differentiation was confirmed by culturing CD34+ cells onto stroma cells engineered to express the physiological membrane-bound Dll4. This blockade was accompanied by a downmodulation of MK transcriptional response. Nevertheless, if Dll4 impaired the generation of mature CD41a+CD42b+ and platelet-forming cells, it did not seem to affect the early steps of MK differentiation, such as the generation of megakaryocytic/erythroid progenitors and CFU-MK, or regulation of some transcription factors involved in early MK differentiation, such as AML1 and GATA1.
This study also shows that the Notch/Dll4 signaling slightly favors, in parallel, erythroid differentiation. We did not observe a strong effect in our culture conditions, but these data are in agreement with a previous study demonstrating that Dll4 promotes erythroid differentiation from human umbilical cord blood CD34+ cells.15 These authors showed an increase in the production of GPA+ cells by exposing human CD34+ cells to Dll4-expressing stroma cells or a recombinant Dll4Fc protein.
No effect of Notch signaling on early steps of human MK differentiation, such as the phenotypically defined erythroid and MK progenitor compartment (MEP) or CFU-MK was evidenced in our culture conditions. This result is at odds with recent results obtained in the mouse model, because Notch signaling has been identified as specifying MK development from hematopoietic stem cells,16,31 especially by increasing the numbers of MEP, CFU-MK, and CD41a+ cells. Our results with murine LSK cells confirm these data and highlight a discrepancy between humans and mice in Notch effect during early steps of MK differentiation. Such interspecies differences have been emphasized in a study comparing documented phenotypes of null mutations in humans and mice.32 By focusing on 120 human genes with clinical features of death before puberty, 22.5% of their mouse orthologs appeared nonessential in mice, such as G-CSF3R or ADAMTS13. Conversely, murine essential genes, nonessential in humans, have been documented, such as humans with homozygous RECQL null alleles, which display viable and fertile Bloom syndrome, whereas targeted deletion of the ortholog in the mouse causes embryonic lethality.33 Thus, homologous proteins might not have equivalent functions in regulatory networks or may not even be part of the same network in different species.
The presently described effects of Dll4/Notch signaling on late steps of MK differentiation are less documented, because most of the studies have focused their attention on earlier steps of megakaryocytopoiesis, without assessing the effects on platelet production. Nevertheless, a previous report has shown the high Dll4 exposure in mice induced a profound thrombocytopenia that could be explained by an inhibitory role of Notch/Dll4 on terminal MK differentiation.11 In fact, although all lineages were affected by a deficit in production, primitive stem cells, B cells, and platelets were the most durable, profoundly affected cell types. Platelet numbers in mice reconstituted with Dll4-producing bone marrow cells never reached more than 20% of control levels during the 17-week survey. Interestingly, although Mercher et al reported that Notch pathway activation led to polyploid MK development both in vivo and in vitro,16 in mice transplanted with intracellular portion of Notch1–transduced progenitors, the proportion of GFP+ platelets was reduced, compared with the proportion of GFP+-transduced bone marrow cells (T. Mercher, personal communication). This observation supports an altered platelet production from intracellular portion of Notch1–expressing MK. Moreover, overexpression of the dominant negative MAML1 in progenitor cells reduced the generation of mature CD42b+ MK (3.6% for control vs 0.8% for dnMAML1), without affecting platelet counts.16 A recent review on Notch signaling in the immune system underlined this observation, leading them to suggest the existence of a different level of regulation for platelet formation.34 In light of all the data established in murine cells, Notch signaling is able to specify MK lineage commitment from LSK cells, but its involvement in terminal MK differentiation is unknown. Overall, Notch signaling in murine MK differentiation can be compared with its activity in T-cell differentiation, with a requirement for the initiation of differentiation, followed by a down-regulation to generate mature T cells.
The blockade in late MK differentiation in human cells was accompanied by a downmodulation of MK transcriptional response. A decrease in NF-E2 and β1-tubulin expression was observed in the few CD41a+CD42b+ cells generated in the presence of Dll4Fc, which is consistent with an inhibition of late MK differentiation. In fact, mice lacking the transcription factor, NF-E2, show profound thrombocytopenia, and their MKs fail to produce proplatelets,30 while β1-tubulin, which is regulated by p45NF-E2, is involved in proplatelet formation.29 FLI-1 expression was weakly, but significantly, down-regulated in response to Dll4Fc, which is also consistent with an inhibition of late MK differentiation, because this factor transcriptionally activates the expression of late MK genes, such as GPIX (CD42a), and GPIbα (CD42b). Furthermore, homologous FLI-1 deletion leads to megakaryopoiesis defects in mice embryos,35 whereas hemizygous loss of FLI-1 is responsible for dysmegakaryopoiesis in human Paris-Trousseau syndrome.36
The transcriptomic response of genes implicated in early MK differentiation showed a transient up-regulation of c-myb and EKLF, both of them being described as regulators of the erythroid-megakaryocytic lineage bifurcation. In fact, gain- and loss-of-function approaches have shown that EKLF inhibits the formation of MK, while, at the same time, stimulating erythroid differentiation.37,38 Analysis of hematopoietic phenotypes in mice bearing hypomorphic c-myb mutations supports the notion that low levels of MYB activity are sufficient for, and even favor, megakaryopoiesis.39 Although transcription of these regulators was down-regulated, no effect on MEP and CFU-MK were evidenced. Nevertheless, the increased levels of c-myb and EKLF transcription, although significant, were very low, compared with studies cited in the previous paragraph, perhaps explaining the absence of detectable effects on early steps of MK differentiation.
In conclusion, this study revealed an inhibitory role of the Notch/Dll4 signaling pathway in human terminal MK differentiation and highlights differences in megakaryocytopoiesis between human and mouse cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The dominant-negative version of MAML-1 was kindly provided by Warren Pear. We also thank the midwives and nurses who helped us to collect cord-blood samples (Maternité des Lilas). We thank F. Larbret and Y. Lécluse for performing the cell sorting, N. Debili for advice for MK cultures, and I. Dusanter-Fourt, D. Duménil, and T. Mercher for critically reading the manuscript. We are indebted to Amgen for providing us with rhu-SCF and FLT3-L and Kirin Brewery Co. for rhuTPO, Novartis for rhu-IL-3, and Cilag for rhu-EPO.
This work was supported by grants from Inserm, Institut Gustave Roussy (Contrat de Recherche Clinique no. 2000.10 and CRI-SPS-2003-02), Association de Recherche contre le Cancer (Grant 4300), Atelier Thérapie Cellulaire Cellules Souches, Ligue Nationale contre le cancer (équipe labellisée WV2010), and Agence Nationale de la recherche (to W.V.).
Authorship
Contribution: J.L.V., W.V., and E.L. designed research; S.P.C., C.C., M.L., and E.L. performed research; S.P.C. and E.L. analyzed data; and E.L., J.L.V., E.S., and W.V. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: E. Lauret, Inserm U1016, Institut Cochin, Department Immuno/Hematologie, Maternité Port-Royal, 5ème étage, 123 Boulevard de Port-Royal 75014 Paris, France; e-mail: evelyne.lauret@inserm.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal