Abstract
The C-type lectin receptor Dectin-1 plays a pivotal role in antifungal immunity. In this study, the recently characterized human DECTIN1 Y238X early stop codon polymorphism leading to diminished Dectin-1 receptor activity was studied in relation to invasive aspergillosis susceptibility and severity in patients receiving hematopoietic stem cell transplantation. We found that the presence of the DECTIN1 Y238X polymorphism in either donors or recipients of hematopoietic stem cell transplantation increased susceptibility to aspergillosis, with the risk being highest when the polymorphism was present simultaneously in both donors and recipients (adjusted hazard ratio = 3.9; P = .005). Functionally, the Y238X polymorphism impaired the production of interferon-γ and interleukin-10 (IL-10), in addition to IL-1β, IL-6, and IL-17A, by human peripheral mononuclear cells and Dectin-1 on human epithelial cells contributed to fungal recognition. Mechanistically, studies on preclinical models of infection in intact or bone marrow-transplanted Dectin-1 knockout mice revealed that protection from infection requires a distinct, yet complementary, role of both donor and recipient Dectin-1. This study discloses Dectin-1 deficiency as a novel susceptibility factor for aspergillosis in high-risk patients and identifies a previously unsuspected role for Dectin-1 in antifungal immunity that is the ability to control both resistance and tolerance to the fungus contingent on hematopoietic/nonhematopoietic compartmentalization.
Introduction
Aspergillus spp are ubiquitous in nature, and the spectrum of diseases they cause is myriad, including saprophytic colonization of preexisting cavities (aspergilloma), allergic asthma, hypersensitivity pneumonitis, allergic bronchopulmonary aspergillosis occurring as a complication of bronchial asthma or cystic fibrosis, and disseminated disease associated with high mortality rates in patients with hematologic malignancies and recipients of solid organs and stem cell transplantations.1 Immunocompetent and nonatopic subjects are relatively resistant to infections, and disease occurs in the setting of host damage.2 The association of persistent inflammation with intractable infection is common in non-neutropenic patients after hematopoietic stem cell transplantation (HSCT) as well as in allergic fungal diseases.2 The current understanding of the pathophysiology underlying Aspergillus infection and disease highlights a truly bipolar nature of the inflammatory process in infection. Early inflammation prevents or limits infection, but an uncontrolled response may eventually oppose disease eradication. This condition is crucially exemplified in mice with chronic granulomatous disease, in which an intrinsic, genetically determined failure to control inflammation to sterile fungal components determines the animals' inability to resolve an actual infection with A fumigatus.3 A main implication of these findings is that, at least in specific clinical settings, it is an exaggerated inflammatory response that probably compromises a patient's ability to eradicate infection, and not an “intrinsic” susceptibility to infection that determines a state of chronic or intractable disease. We have recently found that blocking inflammatory pathways in vivo (ie, PI3K/Akt/mTOR) could be exploited for the development of siRNA therapeutics to attenuate inflammation in aspergillosis.4
A number of studies have highlighted the importance of Dectin-1 in antifungal immunity, in both mice and humans.5-7 Dectin-1 deficiency attenuated the inflammatory response while increasing the lung fungal burden in experimental aspergillosis,8 a finding largely attributed to defective interleukin-17 (IL-17) production. However, although IL-17-producing cells are present in the peripheral T-cell repertoire of humans,9 it has recently been suggested that anti-Aspergillus human host defense relies on Th1, rather than Th17, cellular immunity.10 This suggests that the role of Dectin-1 in antifungal immunity may go beyond the promotion of an inflammatory activity, eventually leading to fungal clearance and antifungal immunity. In this regard, a recent study has shown that, besides the activation of the Syk-dependent canonical transcription factor nuclear factor-κB (NF-κB) to induce antifungal immunity, Dectin-1 also activates the noncanonical NF-κB pathway.11 Given the distinct, yet complementary, role of the canonical/noncanonical NF-κB pathways in immunity and tolerance to Aspergillus,4,12 this finding would predict additional, unanticipated activities of Dectin-1 in antifungal immunity and its regulation.
Recently, genetic polymorphisms affecting human Dectin-1 have also been addressed as potential predictive factors for the incidence of fungal infections. A polymorphism in the human DECTIN1 gene (Y238X, rs16910526) that generates an early stop codon was associated with recurrent mucocutaneous fungal infections in a Dutch family13 and with Candida colonization after HSCT.14 This polymorphism, leading to the loss of the last 10 amino acids of the extracellular domain of Dectin-1, results in impaired transport to the cell surface as well as failure in mediating β-glucan binding.14 Although these studies have focused on the role of the DECTIN1 Y238X polymorphism in modulating susceptibility to Candida, the pathologic aspects of human Dectin-1 deficiency in the immune response to Aspergillus are still to be appreciated.
In this study, we have evaluated the association of the DECTIN1 Y238X polymorphism with invasive aspergillosis (IA) in HSCT patients. A side-by-side comparison of Dectin-1 functional deficiency in humans and mice has disclosed Dectin-1 deficiency as a novel susceptibility factor for aspergillosis in high-risk patients.
Methods
Human study population
A total of 205 patients with hematologic malignancies who underwent T cell–depleted allogeneic HSCT, and of whom stored donor and patient DNA was available, were included in the genetic association study. All transplantations were performed at the Hematopoietic Stem Cell Transplant Unit, Ospedale S Maria della Misericordia, Perugia, Italy, between January 2003 and May 2009. Main patient, disease, and transplantation characteristics are summarized in Table 1. Patients with a history of treated pretransplantation aspergillosis were excluded from the study. Graft processing and transplantation procedures were performed as previously described.15 Grafts consisted of immunoselected CD34+ peripheral blood cells in all cases. No graft-versus-host disease (GVHD) prophylaxis or granulocyte colony-stimulating factor was administered after transplantation. Steroid therapy was administered only to patients who ultimately developed GVHD. The local ethics committee (Comitato Etico delle Aziende Sanitarie dell' Umbria, Perugia) provided institutional review board approval for this study, and informed written consent was obtained from all participants in accordance with the Declaration of Helsinki.
Patient, disease, and transplantation characteristics (N = 205)
| Characteristic . | Value . |
|---|---|
| Median age at transplantation, y (range) | 38 (6-68) |
| Sex, no. (% male) | 103 (50) |
| Sex of donor/patient pair | |
| Female/male | 51 (25) |
| Others | 154 (75) |
| HLA matching, no. (%) | |
| HLA-identical sibling | 69 (34) |
| One HLA haplotype-mismatched family member | 136 (66) |
| Underlying disease, no. (%) | |
| Acute leukemia | 146 (71) |
| Lymphoma/myeloma | 47 (23) |
| Chronic leukemia | 12 (6) |
| Advanced disease stage, no. (%) | 137 (67) |
| Conditioning regimen, no. (%) | |
| With TBI | 153 (75) |
| Without TBI | 52 (25) |
| CMV serology of donor and recipient, no. (%) | |
| CMV−/CMV− | 20 (10) |
| CMV−/CMV+, CMV+/CMV+, or CMV+/CMV− | 185 (90) |
| GVHD, grade II to IV, no. (%) | 11 (5) |
| Invasive aspergillosis, no. (%) | |
| Proven | 19 (9) |
| Probable | 20 (10) |
| Possible | 26 (13) |
| Characteristic . | Value . |
|---|---|
| Median age at transplantation, y (range) | 38 (6-68) |
| Sex, no. (% male) | 103 (50) |
| Sex of donor/patient pair | |
| Female/male | 51 (25) |
| Others | 154 (75) |
| HLA matching, no. (%) | |
| HLA-identical sibling | 69 (34) |
| One HLA haplotype-mismatched family member | 136 (66) |
| Underlying disease, no. (%) | |
| Acute leukemia | 146 (71) |
| Lymphoma/myeloma | 47 (23) |
| Chronic leukemia | 12 (6) |
| Advanced disease stage, no. (%) | 137 (67) |
| Conditioning regimen, no. (%) | |
| With TBI | 153 (75) |
| Without TBI | 52 (25) |
| CMV serology of donor and recipient, no. (%) | |
| CMV−/CMV− | 20 (10) |
| CMV−/CMV+, CMV+/CMV+, or CMV+/CMV− | 185 (90) |
| GVHD, grade II to IV, no. (%) | 11 (5) |
| Invasive aspergillosis, no. (%) | |
| Proven | 19 (9) |
| Probable | 20 (10) |
| Possible | 26 (13) |
Microbiologic and clinical evaluation
Antifungal prophylaxis included liposomal amphotericin-B (1 mg/kg daily) in high-risk patients and fluconazole (400 mg daily) in standard-risk patients from day −5 until neutropenia ended. The criteria defining high risk for invasive fungal disease at the time of allogeneic HSCT were as follows: (1) invasive fungal disease before receipt of transplantation (these patients have been excluded from the study), (2) HSCT from a human leukocyte antigen (HLA) haplotype-mismatched family donor, and (3) HSCT from an HLA-identical sibling, if conditioning included total body irradiation (TBI).16 Surveillance cultures for fungal growth from stool, urine, nasal, and oral washes were performed twice weekly after transplantation. Surveillance also included the determination of blood galactomannan (Platelia Aspergillus EIA, Bio-Rad), with positivity being observed in 21 (54%) patients with IA. In addition, blood, sputum, bronchoalveolar lavage (BAL), and cultures of samples from infected sites were analyzed when clinical signs and/or symptoms of infection appeared. Positivity of microbiologic tests with clinical or instrumental signs of fungal infection was considered indicative of probable/proven infection according to the revised standard criteria from the European Organization for Research and Treatment of Cancer/Mycology Study Group,17 in which case, conventional antifungal treatment was started. The median time to IA after HSCT was 97 days (range, 1-390 days). Among the 205 patients enrolled in the study, 39 (19%) developed proven or probable IA, whereas patients with possible fungal infection (26 of 205, 13%) were excluded from the analysis (Table 1). Localization of IA included lung (n = 30, 77%) and sinus (n = 2, 5%), whereas disseminated infection occurred in 7 patients (18%). Among the patients with IA, 4 (10%) also developed GVHD.
Genetic screening of the DECTIN1 Y238X polymorphism
Genomic DNA from whole blood samples from patients and donors was isolated before transplantation using the QIAamp DNA Blood Mini kit (QIAGEN) following the manufacturer's instructions and stored at −20°C. The DECTIN1 Y238X polymorphism (rs16910526) was selected based on previously published evidence of association with susceptibility to Candida colonization and infection.13,14 Genotyping was performed using bidirectional polymerase chain reaction (PCR) amplification of specific alleles (Bi-PASA), as previously described.18 The PCR primers used were as follows: P1, GTAGTCCCAGCTACTTGAGG; P2, ACCACTTGAGATTCACAACA; P3, ggcggcggggAGTGTGCCCTCATAT; and P4, gggccgggggTTCTTCTCACAAATACTC. Allele and genotype frequencies of the DECTIN1 Y238X polymorphism were similar to those observed in the National Center for Biotechnology Information database and comparable between donors and recipients (data not shown). Regarding quality control, each PCR set was composed of randomly selected replicates of previously typed samples from the study and 2 negative controls. Agreement for quality control and duplicates was more than or equal to 99% for all assays. Laboratory personnel were blind to the sample status.
Mice
Female C57BL/6 and BALB/c mice, 8 to 10 weeks old, were purchased from Charles River Laboratories. These strains were selected on the consideration that BALB/c mice present comparable levels of both Dectin-1A and Dectin-1B transcripts, whereas C57BL/6 mice predominantly express the smaller Dectin-1B isoform.19 Homozygous Dectin-1-deficient mice on both the C57BL/6 and BALB/c backgrounds (kindly supplied by Emiko Kazama, University of Tokyo, Japan) were bred under specific pathogen-free conditions at the Animal Facility of Perugia University, Perugia, Italy. Experiments were performed according to the Italian Approved Animal Welfare Assurance A-3143-01.
Fungal strain and infection
The strain of A fumigatus was obtained from a fatal case of pulmonary aspergillosis at the Infectious Diseases Institute of the University of Perugia. Viable conidia (> 95%) were obtained by growth on Sabouraud dextrose agar (Difco Laboratories) supplemented with chloramphenicol for 4 days at room temperature. Inactivated resting or swollen conidia were obtained as described.20 For infection, mice were anesthetized by intraperitoneal injection of 2.5% avertin (Sigma-Aldrich) before instillation of a suspension of 2 × 107 conidia/20 μL saline intratracheally. Fungi were suspended in endotoxin-free (Detoxi-gel; Pierce Chemical) solutions (< 1.0 EU/mL, as determined by the Limulus amebocyte lysate method). Mice were monitored for fungal growth (colony-forming units [CFU]/organ, mean ± SE) and lung histology (periodic acid-Schiff [PAS] staining). BAL was performed by cannulating the trachea and washing the airways with 3 mL of phosphate-buffered saline to collect the BAL fluid. Total and differential cell counts were done by staining BAL smears with May-Grünwald-Giemsa reagents (Sigma-Aldrich) before analysis. At least 200 cells per cytospin preparation were counted, and the absolute number of each cell type was calculated.
Generation of HSCT mice
Bone marrow cells from C57BL/6 or BALB/c donor mice were resuspended in Hanks balanced salt solution (Invitrogen) containing 10% fetal bovine serum (Invitrogen) at a concentration of 100 × 106 cells/mL. T-cell depletion was obtained on incubation of the cells with MicroBeads (Miltenyi Biotec) conjugated to rat antimouse CD5 monoclonal antibody (Ly-1; clone 53-7.3) for 15 minutes at 4°C followed by magnetic sorting in a magnetic cell separator (CliniMACS cell separation system, Miltenyi Biotec). Unbound cells were carefully aspirated, washed, and resuspended at a concentration of 100 × 106 cells/mL. Recipient C57BL/6 or BALB/c mice were exposed to a lethal dose of 9 Gy, infused with T cell-depleted donor cells, and infected with Aspergillus conidia a week later.9
Cytokine production by human PBMCs
On written informed consent, human peripheral blood mononuclear cells (PBMCs) isolated from healthy blood donors bearing the wild-type (WT) DECTIN1 (n = 8) or the heterozygous Y238X allele (n = 4) were stimulated with heat-inactivated A conidia, β-glucan (5 μg/mL),9 or phytohemagglutinin for 72 hours before determination of cytokine content in the culture supernatant.21
siRNA-mediated DECTIN1 silencing in human epithelial cells
The human bronchial epithelial BEAS-2B cells were cultured as described.22 After cell culture in 24-well plates (∼ 60%–80% confluence), BEAS-2B cells were transfected with a nonspecific scrambled siRNA and with a DECTIN1-specific siRNA (siDectin-1) at a final concentration of 10nM (TriFECTa Dicer-Substrate screening RNAi kit; IDT, Tema Ricerca Srl) using the TransIT-siQuest transfection reagent (Mirus Bio) according to the manufacturer's instructions. Transfection efficiencies were determined with a positive fluorescent-labeled transfection control siRNA, and the silencing effects of siRNAs were confirmed by RT-PCR. Forty-eight hours after transfection, cells were stimulated overnight with heat-inactivated A conidia at 1:1 cells/fungi ratio or 5 μg/mL β-glucan, before total cellular RNA isolation and culture supernatants collection.
ELISA and real-time PCR
The level of cytokines in culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA; SearchLight Custom Array; Aushon Biosystems, Tema Ricerca Srl). Real-time RT-PCR was performed using the iCycler iQ detection system (Bio-Rad) and SYBR Green chemistry (Finnzymes). Cells were lysed and total RNA was extracted using RNeasy Mini Kit (QIAGEN) and were reverse transcribed with Sensiscript Reverse Transcriptase (QIAGEN) according to the manufacturer's directions. The PCR primers were as described.23 Amplification efficiencies were validated and normalized against Gapdh. The thermal profile for SYBR Green real-time PCR was at 95°C for 3 minutes, followed by 40 cycles of denaturation for 30 seconds at 95°C and an annealing/extension step of 30 seconds at 60°C. Each data point was examined for integrity by analysis of the amplification plot. The mRNA-normalized data were expressed as relative cytokine mRNA in stimulated cells compared with that of mock-infected cells.
Flow cytometry
Membrane-bound Dectin-1 expression in monocytes was determined by flow cytometry (FACSCanto; BD Biosciences). Briefly, whole blood samples were incubated with 10 μg/mL fluorescein isothiocyanate-conjugated mouse antihuman CD14 monoclonal antibody (clone MϕP9; BD Biosciences PharMingen) and 5 μg/mL phycoreythrin-conjugated mouse antihuman Dectin-1 monoclonal antibody (clone 259931; R&D Systems) or fluorochrome-conjugated isotype controls, according to the manufacturer's instructions.
Statistical analysis
Consistency of genotype frequencies with the Hardy-Weinberg equilibrium was tested using a χ2 test on a contingency table of observed versus predicted genotype frequencies (P > .05). The probability of IA was compared using the Gray test24 and analyzed using the cumulative incidence method. Cumulative incidences were computed with the cmprsk package for R, Version 2.10.1 software,25 with the competing events for IA being relapse and death. Overall survival (OS) was defined as the time from first day of treatment to death from any cause and was obtained by the Kaplan-Meier method and compared using the log-rank test. Variables included in the univariate analyses were: donor and patient DECTIN1 Y238X genotype, patient age (younger vs older than the median), patient gender, relation donor-patient gender (donor female into recipient male vs others), disease stage (advanced vs early), transplantation matching (HLA-identical sibling vs 1 HLA haplotype-mismatched family member), killer immunoglobulin-like receptor genotype (matched vs mismatched), cytomegalovirus (CMV) serology of donors and patients (donor CMV− and patient CMV− vs others), and conditioning regimen (with TBI vs absence of TBI). GVHD was considered in the univariate analysis as a time-dependent covariate for IA. All prognostic variables in the univariate analysis with a P value less than or equal to .15, as well as patient age and sex, were included in the multivariate analysis. Multivariate analysis was performed using the subdistribution regression model of Fine and Gray26 with the cmprsk package for R, Version 2.10.1 software.27 The overall P value for the 3-level genetic model was obtained through the Wald test. Animal data were analyzed by GraphPad Prism, Version 4.03 program (GraphPad Software). Student t test or analysis of variance and Bonferroni test were used to determine the statistical significance (P) of differences in organ clearance and in vitro assays. The data reported are either from 1 representative experiment of 3 to 5 independent experiments (RT-PCR) or pooled from 3 to 5 experiments, otherwise. The in vivo groups consisted of 6 to 8 mice/group.
Results
DECTIN1 Y238X polymorphism affects susceptibility to invasive aspergillosis
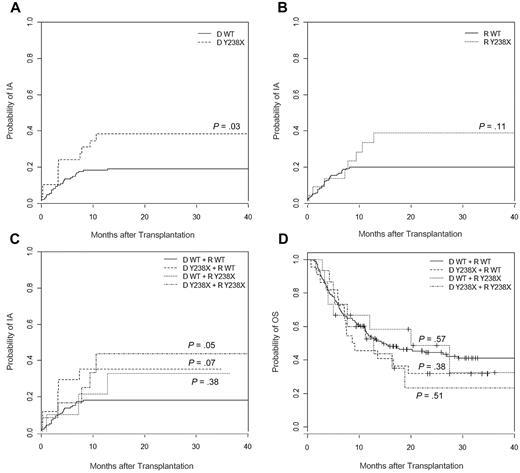
To assess the risk of IA according to the DECTIN1 Y238X polymorphism in recipients and donors of HSCT, we estimated the cumulative incidence of IA at 6 and 36 months after transplantation. We found that donor Y238X polymorphism was associated with IA at 36 months (cumulative incidence, 38% in the presence of the allele vs 19% in its absence; P = .03), although a trend toward an increased risk of IA was also observed at 6 months (Figure 1A). Interestingly, recipient Y238X polymorphism also tended to influence the risk of IA at 36 months (cumulative incidence, 39% in the presence of the allele vs 20% in its absence; P = .11), although in this case, no differences were observed at 6 months after transplantation (Figure 1B). The fact that risk of IA at 36 months was further increased when the Y238X polymorphism was present simultaneously in both recipients and donors (cumulative incidence, 44% for Y238X pairs vs 18% for WT pairs; P = .05; Figure 1C) not only suggests an apparent gene-dosage effect but also points to a distinct, yet complementary, role of donor and recipient Dectin-1 in the overall antifungal resistance in transplantation setting. Accordingly, an intermediate risk of IA was observed for both Y238X/WT and WT/Y238X donor/recipient pairs (cumulative incidence, 35%; P = .07, and 33%; P = .38, respectively; Figure 1C).
Cumulative incidence of invasive aspergillosis and OS estimate at 36 months after HSCT according to DECTIN1 Y238X genotype. (A) Cumulative incidence according to donor genotype: WT (19%, N = 150, reference) and Y238X (38%, N = 29; P = .03). (B) Cumulative incidence according to recipient genotype: WT (20%, N = 157; reference) and Y238X (39%, N = 22; P = .11). (C) Cumulative incidence according to donor/recipient pair genotype: WT (18%, N = 140; reference), donor Y238X and recipient WT (35%, N = 17; P = .07), donor WT and recipient Y238X (33%, N = 10; P = .38), and Y238X (44%, N = 12; P = .05). (D) OS according to donor/recipient pair genotype: WT (41%, N = 153; reference), donor Y238X and recipient WT (32%, N = 22; P = .38), donor WT and recipient Y238X (32%, N = 15; P = .57), and Y238X (23%, N = 15; P = .51).
Cumulative incidence of invasive aspergillosis and OS estimate at 36 months after HSCT according to DECTIN1 Y238X genotype. (A) Cumulative incidence according to donor genotype: WT (19%, N = 150, reference) and Y238X (38%, N = 29; P = .03). (B) Cumulative incidence according to recipient genotype: WT (20%, N = 157; reference) and Y238X (39%, N = 22; P = .11). (C) Cumulative incidence according to donor/recipient pair genotype: WT (18%, N = 140; reference), donor Y238X and recipient WT (35%, N = 17; P = .07), donor WT and recipient Y238X (33%, N = 10; P = .38), and Y238X (44%, N = 12; P = .05). (D) OS according to donor/recipient pair genotype: WT (41%, N = 153; reference), donor Y238X and recipient WT (32%, N = 22; P = .38), donor WT and recipient Y238X (32%, N = 15; P = .57), and Y238X (23%, N = 15; P = .51).
The clinical risk factors associated or with a trend toward IA in the univariate analysis were to receive an HLA haplotype-mismatched transplantation (27% vs 15% at 36 months; P = .03) and to develop GVHD (44% vs 21% at 36 months; P = .12; data not shown). Therefore, the final multivariate model, in addition to patient age and sex, included DECTIN1 Y238X allele combinations in donor/recipient pairs, HLA haplotype-mismatching, and GVHD (Table 2). The Y238X/Y238X allele combination resulted in a 3.9-fold increased risk of developing IA after transplantation (adjusted hazard ratio = 3.89; 95% confidence interval, 1.51-9.99; P = .005), whereas Y238X/WT and WT/Y238X donor/recipient allele combinations led to 2.5- (adjusted hazard ratio = 2.50; 95% confidence interval, 1.00-6.53; P = .05) and 1.5-fold increased risk (adjusted hazard ratio = 1.51; 95% confidence interval, 0.46-4.93; P = .49), respectively (Table 2).
Multivariate analysis of the association of the DECTIN1 Y238X polymorphism with risk of invasive aspergillosis
| Genetic/clinical risk factors* . | Adjusted HR . | 95% CI . | P . |
|---|---|---|---|
| D Y238X + R WT | 2.50 | 1.00-6.53 | .05 |
| D WT + R Y238X | 1.51 | 0.46-4.93 | .49 |
| D Y238X + R Y238X | 3.89 | 1.51-9.99 | .005 |
| HLA haplotype mismatch | 2.70 | 1.31-5.56 | .007 |
| GVHD | 3.10 | 1.10-8.71 | .03 |
| Genetic/clinical risk factors* . | Adjusted HR . | 95% CI . | P . |
|---|---|---|---|
| D Y238X + R WT | 2.50 | 1.00-6.53 | .05 |
| D WT + R Y238X | 1.51 | 0.46-4.93 | .49 |
| D Y238X + R Y238X | 3.89 | 1.51-9.99 | .005 |
| HLA haplotype mismatch | 2.70 | 1.31-5.56 | .007 |
| GVHD | 3.10 | 1.10-8.71 | .03 |
D indicates donor; and R, recipient.
For the genetic factors, D WT + R WT genotype combination is the referent category; overall P value for the three-level DECTIN1 Y238X genotype is P = .019.
A total of 117 (57%) patients died after transplantation, leaving 88 (43%) long-term survivors. Median follow-up time among patients who survived was 28 months (range, 5-77 months) and among those who died was 6 months (range, 1-57 months). Although no statistically significant differences were observed, the probability of survival at 36 months was nevertheless found to decrease from 41% among patients from WT pairs to 23% among patients with the high-risk Y238X/Y238X allele combination (Figure 1D).
DECTIN1 Y238X polymorphism associates with defective Dectin-1 activity
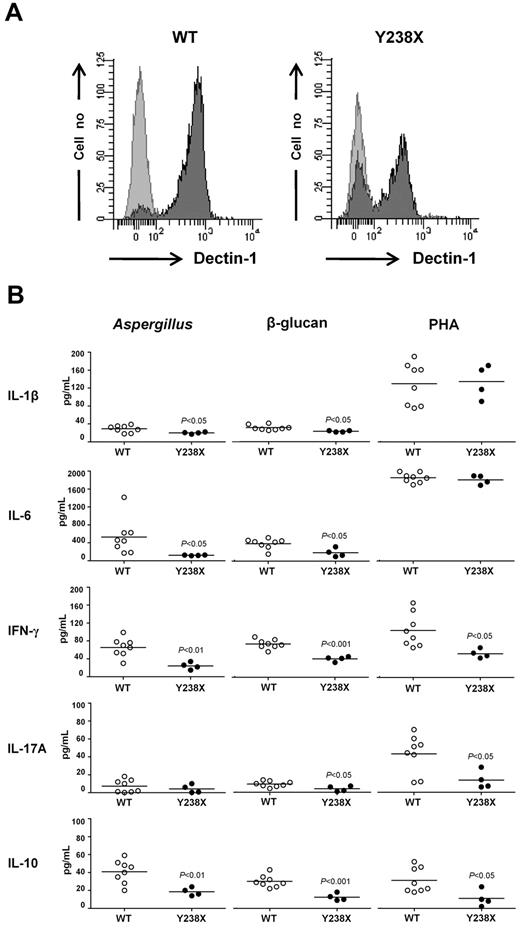
To investigate whether the DECTIN1 Y238X polymorphism associates with decreased surface expression of Dectin-1 and altered cytokine production in response to specific ligands, as already reported,13,14 we first analyzed membrane-bound Dectin-1 expression in gated CD14+ peripheral monocytes from WT (n = 8) or Y238X (n = 4) heterozygous persons by flow cytometry. We found that unstimulated CD14+ monocytes from Y238X persons display a decreased surface expression of Dectin-1 (76% ± 5%; range, 62%–83%) compared with WT monocytes (91% ± 1%; range, 89%–93%; P = .02; Figure 2A). Dectin-1 mRNA expression was instead identical between genotypes (data not shown). Functionally, in response to β-glucan or A conidia, PBMCs isolated from persons heterozygous for the Y238X polymorphism showed a decreased production of IL-1β and IL-6 (Figure 2B), as already reported,13,14 whereas no significant variations were observed in the production of tumor necrosis factor-α, CCL3/macrophage inflammatory protein-1α, or CCL4/macrophage inflammatory protein-1β (data not shown). Interestingly, the results showed that interferon-γ (IFN-γ) and IL-10, more than IL-17A, productions were also decreased in PBMCs harboring the Y238X polymorphism in response to all stimuli, including phytohemagglutinin (Figure 2B), whereas IL-4 was always below the detection limit (data not shown). These results indicate that Dectin-1 not only regulates the production of inflammatory cytokines, such as IL-1β, IL-6, and IL-17A, in response to Aspergillus but also the production of IFN-γ/IL-10, both cytokines playing a pivotal role in the protective immune responses to the fungus.4,28
DECTIN1 Y238X polymorphism associates with defective Dectin-1 activity. (A) Surface Dectin-1 expression in unstimulated CD14+ monocytes from WT or heterozygous persons for the DECTIN1 Y238X polymorphism. Dectin-1 expression was assessed in 8 WT and 4 Y238X heterozygous persons. Representative flow cytometry graphs of extracellular Dectin-1 staining (dark gray peak) and isotype control (light gray peak) are shown. (B) Cytokine production by human PBMCs from WT or heterozygous persons for the DECTIN1 Y238X polymorphism. Cytokine production (picograms per milliliter) was quantified by ELISA on culture supernatants of cells stimulated with heat-inactivated A conidia, β-glucan or phytohemagglutinin for 72 hours. Shown are the results of 8 WT and 4 Y238X polymorphic persons, assessed in triplicates. The detection limit of the assays was less than 0.5 pg/mL for each cytokine.
DECTIN1 Y238X polymorphism associates with defective Dectin-1 activity. (A) Surface Dectin-1 expression in unstimulated CD14+ monocytes from WT or heterozygous persons for the DECTIN1 Y238X polymorphism. Dectin-1 expression was assessed in 8 WT and 4 Y238X heterozygous persons. Representative flow cytometry graphs of extracellular Dectin-1 staining (dark gray peak) and isotype control (light gray peak) are shown. (B) Cytokine production by human PBMCs from WT or heterozygous persons for the DECTIN1 Y238X polymorphism. Cytokine production (picograms per milliliter) was quantified by ELISA on culture supernatants of cells stimulated with heat-inactivated A conidia, β-glucan or phytohemagglutinin for 72 hours. Shown are the results of 8 WT and 4 Y238X polymorphic persons, assessed in triplicates. The detection limit of the assays was less than 0.5 pg/mL for each cytokine.
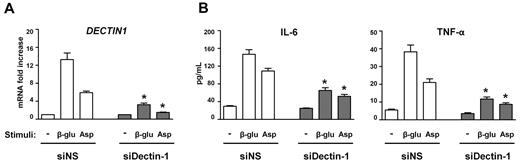
To gain insights into the possible consequences the DECTIN1 Y238X polymorphism may have on cells beyond the hematopoietic compartment, we resorted to respiratory epithelial cells, known to sense A fumigatus and participate in the host defense.29 Dectin-1 mRNA and protein expression is known to be inducible on human airway epithelial cells.30 Thus, we assessed Dectin-1 mRNA expression and cytokine production in BEAS-2B cells on stimulation with β-glucan or A conidia and the effects of Dectin-1 blockade by siRNA. We found that both β-glucan and A conidia greatly induced Dectin-1 mRNA (Figure 3A) and concomitantly the production of IL-6 and tumor necrosis factor-α (Figure 3B) in BEAS-2B cells. However, transfection of cells with a Dectin-1-specific siRNA abrogated both these effects (Figure 3A-B). These results suggest that Dectin-1 on epithelial cells is inducible in infection and contribute to the overall cytokine production.
siRNA-mediated silencing of Dectin-1 in human epithelial cells. (A) Dectin-1 mRNA expression in BEAS-2B cells left untreated or stimulated with β-glucan (β-glu) or heat-inactivated A conidia (Asp) after 48 hours of transfection with a Dectin-1-specific siRNA (siDectin-1) or a nonspecific control siRNA (siNS). Transfection efficiencies were determined with a positive fluorescent-labeled transfection control siRNA, and the silencing effects of siRNAs were confirmed by RT-PCR. Results shown were assessed in triplicate. (B) Cytokine production (picograms per milliliter) on culture supernatants of transfected BEAS-2B cells stimulated overnight with either β-glucan or heat-inactivated A conidia. Cytokines were quantified by ELISA, and the results shown were assessed in triplicate. The detection limit of the assays was less than 0.5 pg/mL for each cytokine.
siRNA-mediated silencing of Dectin-1 in human epithelial cells. (A) Dectin-1 mRNA expression in BEAS-2B cells left untreated or stimulated with β-glucan (β-glu) or heat-inactivated A conidia (Asp) after 48 hours of transfection with a Dectin-1-specific siRNA (siDectin-1) or a nonspecific control siRNA (siNS). Transfection efficiencies were determined with a positive fluorescent-labeled transfection control siRNA, and the silencing effects of siRNAs were confirmed by RT-PCR. Results shown were assessed in triplicate. (B) Cytokine production (picograms per milliliter) on culture supernatants of transfected BEAS-2B cells stimulated overnight with either β-glucan or heat-inactivated A conidia. Cytokines were quantified by ELISA, and the results shown were assessed in triplicate. The detection limit of the assays was less than 0.5 pg/mL for each cytokine.
Dectin-1 regulates both resistance and inflammation to Aspergillus in mice
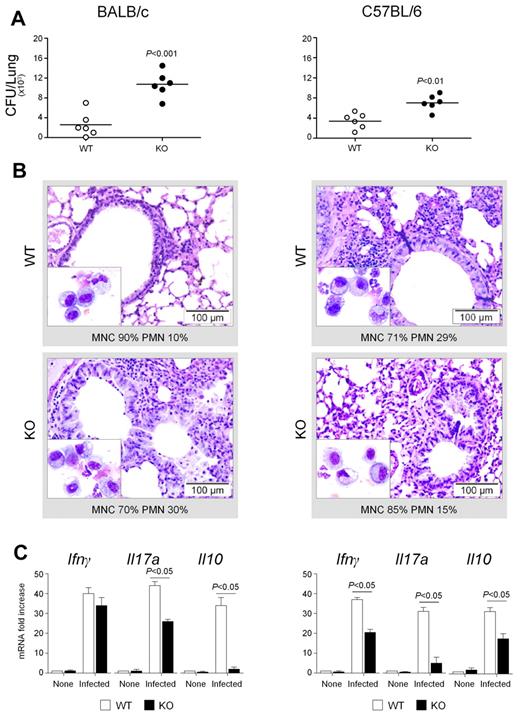
To understand cellular and molecular immune pathways by which the DECTIN1 Y238X polymorphism may affect susceptibility to aspergillosis in HSCT, we resorted to experimental models of pulmonary aspergillosis in which the relative contributions of inflammatory and noninflammatory cytokine pathways are well characterized.3,4,8,20 We injected WT and Dectin-1 knockout (KO) mice on both the BALB/c and C57BL/6 backgrounds with Aspergillus dormant conidia intratracheally and evaluated parameters of susceptibility and resistance to the infection in terms of mortality, fungal growth, inflammatory pathology, and cytokine production. The results show that the fungal burden was higher in KO than WT mice on both backgrounds (Figure 4A), even though the infection was eventually cleared in both types of mice and all survived the infection (data not shown). In terms of inflammatory cell recruitment in the BAL and lung histopathology, differences were observed between WT and KO on the 2 backgrounds (Figure 4B). The inflammatory cell recruitment and lung pathology were greatly increased in KO, compared with WT, mice on the BALB/c background, and this was associated with reduced levels of lung IL-10 and, to a lesser extent, of IFN-γ and IL-17A production (Figure 4C). In contrast, the inflammatory cell recruitment and lung pathology were less pronounced in KO than WT mice on the C57BL/6 background, and this was associated with decreased levels of IFN-γ and IL-17A but less of IL-10 (Figure 4C). Confirming the human data, these results indicate that the functional activity of Dectin-1 in infection includes a regulatory function of IL-10 and IFN-γ, in addition to IL-17A, production and is apparently contingent on host genetic background.
Dectin-1 regulates both resistance and inflammation to Aspergillus. Dectin-1-deficient mice on both BALB/c and C57BL/6 backgrounds were given a suspension of 2 × 107 conidia/20 μL saline intratracheally. (A) Fungal growth (CFU ± SE), (B) lung histology (PAS staining) and BAL morphometry, and (C) cytokine gene expression in the lungs by real-time RT-PCR were assessed 3 days after the infection. For histology, paraffin-embedded tissues (3-4 μm) of lung were stained and mounted with Eukitt mounting medium (Sigma-Aldrich). Histology sections were observed with 40×/0.65 objective lens using an Olympus BX51 microscope (Olympus). BAL cystospins were performed as described in “Methods.” Cytospin preparations were observed with a 100×/1.25 NA oil objective lens using a Olympus BX51 microscope (Olympus). All images were captured using a high-resolution Olympus DP71 camera (Olympus) and digitally acquired using Cell⋀P software (Version 3.3 build 2108, Olympus). Subsequent image editing was performed using Adobe Photoshop CS3 (Version 10.0, Adobe Systems Incorporated). Note the sustained parenchymal damage (PAS staining) and inflammatory cell recruitment in BAL (May-Grünwald-Giemsa staining in the inset) in BALB/c Dectin-1 KO more than C57BL/6 Dectin-1 KO, mice. Bars represent magnifications. The numbers refer to percentage polymorphonuclear (PMN) or mononuclear (MNC) cells on BAL. Data are pooled from 4 experiments or representative of 2 experiments (for histology).
Dectin-1 regulates both resistance and inflammation to Aspergillus. Dectin-1-deficient mice on both BALB/c and C57BL/6 backgrounds were given a suspension of 2 × 107 conidia/20 μL saline intratracheally. (A) Fungal growth (CFU ± SE), (B) lung histology (PAS staining) and BAL morphometry, and (C) cytokine gene expression in the lungs by real-time RT-PCR were assessed 3 days after the infection. For histology, paraffin-embedded tissues (3-4 μm) of lung were stained and mounted with Eukitt mounting medium (Sigma-Aldrich). Histology sections were observed with 40×/0.65 objective lens using an Olympus BX51 microscope (Olympus). BAL cystospins were performed as described in “Methods.” Cytospin preparations were observed with a 100×/1.25 NA oil objective lens using a Olympus BX51 microscope (Olympus). All images were captured using a high-resolution Olympus DP71 camera (Olympus) and digitally acquired using Cell⋀P software (Version 3.3 build 2108, Olympus). Subsequent image editing was performed using Adobe Photoshop CS3 (Version 10.0, Adobe Systems Incorporated). Note the sustained parenchymal damage (PAS staining) and inflammatory cell recruitment in BAL (May-Grünwald-Giemsa staining in the inset) in BALB/c Dectin-1 KO more than C57BL/6 Dectin-1 KO, mice. Bars represent magnifications. The numbers refer to percentage polymorphonuclear (PMN) or mononuclear (MNC) cells on BAL. Data are pooled from 4 experiments or representative of 2 experiments (for histology).
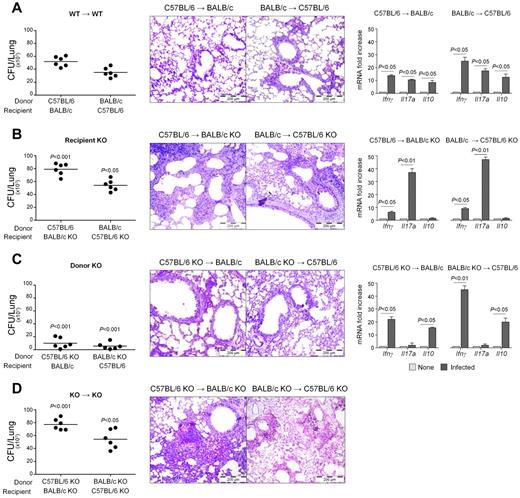
Dectin-1 deficiency affects immunity and tolerance to Aspergillus in HSCT mice
To assess the translational potential of the regulatory activity of Dectin-1 on cytokine production in aspergillosis, we evaluated Th immunity and tolerance to Aspergillus in an experimental model of HSCT in which Dectin-1 KO mice on both genetic backgrounds were used as either donors or recipients or both. As in humans, the susceptibility to the infection increased in conditions of Dectin-1 deficiency. In particular, an unrestricted fungal growth associated with an exuberant inflammatory response and IL-17A production occurred in recipient KO mice, especially on the BALB/c background, in which IFN-γ, but particularly, IL-10 productions were greatly reduced (Figure 5B) compared with recipient WT mice (Figure 5A). The susceptibility to the infection was not increased in donor KO-transplanted mice in which a predominant IFN-γ/IL-10 over IL-17A response was observed (Figure 5C), a finding suggesting a possible compensatory role for recipient Dectin-1. Indeed, in the condition in which both donor and recipient Dectin-1 KO were used, mice experienced the most severe form of infection, both in terms of unrestricted fungal growth and lung inflammation (Figure 5D). These data confirm the crucial role of Dectin-1 in infection and suggest that the contribution of this receptor to antifungal resistance is contingent on hematopoietic/nonhematopoietic compartmentalization. Dectin-1 on donor cells appears to provide antifungal resistance through IL-17A while on recipients through IFN-γ/IL-10.
Dectin-1 deficiency affects immunity and tolerance to Aspergillus in HSCT mice. Dectin-1 KO mice on both genetic backgrounds were used as (B) recipients, (C) donors, or (D) both in an experimental model of HSCT in which lethally irradiated recipient mice are infused with T cell-depleted donor cells and infected with A conidia intratracheally a week later. Fungal growth (CFU ± SE), lung histology (PAS staining; bars represent magnifications), and cytokine gene expression in the lungs by real-time RT-PCR were assessed 3 days after the infection. None indicates uninfected transplanted mice. For histology, paraffin-embedded tissues (3-4 μm) of lung were stained and mounted with Eukitt mounting medium (Sigma-Aldrich). Histology sections were observed under a 20×/0.40 objective lens using an Olympus BX51 microscope (Olympus). All images were captured using a high-resolution Olympus DP71 camera (Olympus) and digitally acquired using Cell⋀P software (Version 3.3 build 2108, Olympus). Subsequent image editing was performed using Adobe Photoshop CS3 (Version 10.0, Adobe Systems Incorporated). Note the exuberant inflammatory cell responses in the lungs of recipient KO and of both donor/recipient KO as opposed to the mild inflammatory parenchymal reaction observed in (A) control WT mice or (C) donor KO. Data are representative of 2 experiments.
Dectin-1 deficiency affects immunity and tolerance to Aspergillus in HSCT mice. Dectin-1 KO mice on both genetic backgrounds were used as (B) recipients, (C) donors, or (D) both in an experimental model of HSCT in which lethally irradiated recipient mice are infused with T cell-depleted donor cells and infected with A conidia intratracheally a week later. Fungal growth (CFU ± SE), lung histology (PAS staining; bars represent magnifications), and cytokine gene expression in the lungs by real-time RT-PCR were assessed 3 days after the infection. None indicates uninfected transplanted mice. For histology, paraffin-embedded tissues (3-4 μm) of lung were stained and mounted with Eukitt mounting medium (Sigma-Aldrich). Histology sections were observed under a 20×/0.40 objective lens using an Olympus BX51 microscope (Olympus). All images were captured using a high-resolution Olympus DP71 camera (Olympus) and digitally acquired using Cell⋀P software (Version 3.3 build 2108, Olympus). Subsequent image editing was performed using Adobe Photoshop CS3 (Version 10.0, Adobe Systems Incorporated). Note the exuberant inflammatory cell responses in the lungs of recipient KO and of both donor/recipient KO as opposed to the mild inflammatory parenchymal reaction observed in (A) control WT mice or (C) donor KO. Data are representative of 2 experiments.
Discussion
Although natural immunity ensures survival of the species as a whole, persons themselves are probably not immunocompetent to all pathogens, and individual differences in susceptibility to specific pathogens are quite common.31 Several polymorphisms in innate immune receptors, most remarkably Toll-like receptors, have been described to influence susceptibility to aspergillosis in distinct settings of patients.32-35 In the present study, we found that the functional Y238X polymorphism in DECTIN1 increased susceptibility to IA among HSCT patients, a finding consistent with the role of Dectin-1-mediated mechanisms of host defense against Aspergillus.5,8,36 The increased susceptibility to aspergillosis underlined by the Y238X polymorphism was found to rely on both donor and recipient genetic make-ups, with the effect being more prominent in conditions in which both donors and recipients simultaneously harbored the Y238X polymorphism. In addition, donor Y238X polymorphism appears to increase the risk of either early (0-30 days) or late (30-120 days) infection, although the impact of recipient Y238X occurs on very late periods after transplantation (after 120 days). Although no significant impact of the Y238X polymorphism was demonstrated on OS, a trend toward a poorer prognosis was nevertheless observed for persons belonging to the high-risk group of Y238X/Y238X donor/recipient pairs. Because no impact of the polymorphism was observed on relapse or GVHD (data not shown), the worsened survival is most probably correlated with the fact that these patients have the highest risk of developing aspergillosis.
The DECTIN1 Y238X polymorphism generates a truncated Dectin-1 protein unable to target the membrane and mediate β-glucan recognition, therefore resulting in defective cytokine production on receptor engagement.13,14 Although Dectin-1 has been regarded as one major innate receptor leading to Th17 activation in response to Aspergillus,8 and the Y238X polymorphism was associated with impaired IL-17 production in response to Candida albicans or β-glucan37 and increased Candida colonization in HSCT patients,13 we found that IFN-γ and IL-10, in addition to IL-1β, IL-6, and IL-17A, productions by PBMCs heterozygous for the Y238X polymorphism were defective on β-glucan- or Aspergillus-specific stimulation. Results obtained with Dectin-1 KO mice confirmed the regulatory activity of Dectin-1 over IFN-γ and IL-10, more than IL-17A, production. These findings are consistent with the recent observations that multiple, yet distinct, fungal molecules other than β-glucan are endowed with the ability to activate human Th17 cells.9 Thus, our study reveals a previously unsuspected role for Dectin-1 in antifungal immunity, which is the ability to modulate immunity and tolerance via IFN-γ/IL-10 production, both cytokines reflecting the activation of protective Th1/Treg antifungal responses in mice4 and humans.10
We have recently shown that the induction of antifungal Th1/Tregs occurs through the noncanonical NF-κB pathway on dendritic cells (DCs),4 a pathway also activated by Dectin-1.11 Therefore, it is tempting to speculate that, by affecting the balance between canonical/noncanonical NF-κB activation on DCs, Dectin-1 may affect the inflammatory state in infection. However, the high risk of infection seen in condition of recipient Dectin-1 deficiency, both in humans and murine models, also points to a crucial role for Dectin-1 expressed on nonhematopoietic cells in the induction of immune protection to the fungus. We found that Dectin-1 was inducible on human epithelial cells and contributes to cytokine production in response to the fungus. Evidence suggests that the airway epithelial cells are not simply “innocent bystanders” but may play a crucial role in innate and inflammatory responses38 and in regulation of antimicrobial immunity.39 Of great interest is the recent finding that the antifungal activity of epithelial cells occurs through signal transduction pathways leading to the induction of the indoleamine 2,3-dioxygenase enzyme,40,41 known to play a pivotal role in regulation of pathogenic inflammatory responses to the fungus.3
In conclusion, our findings highlight the multiple roles Dectin-1 may have in host resistance to Aspergillus that is probably achieved through distinct, yet complementary, mechanisms of immune resistance and tolerance that are dependent on the hematopoietic/nonhematopoietic compartmentalization. This probably accounts for the increased risk of infection in the condition in which the Y238X polymorphism was present simultaneously in both recipients and donors, although a gene-dosage effect cannot be excluded, as recently suggested.42 Moreover, a further layer of complexity may be contingent on host genetic background, as clearly indicated by the results obtained with mouse strains of different genetic backgrounds in which the relative contribution of the full-length Dectin-1A and the stalkless Dectin-1B isoforms is different.19 We have already disclosed polymorphisms in a number of innate immune genes that modulate susceptibility to IA in T cell-depleted transplanted patients.33,43 In addition to the mechanistic insights regarding Dectin-1 function in aspergillosis, the present study further points to the utility of the genetic screening to identify patients at high risk for IA and, whenever possible, for adequate donor selection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cristina Massi Benedetti for digital art and editing and Emiko Kazama, University of Tokyo, Japan, for the provision of breeding pairs of Dectin-1 KO mice.
This work was supported by the Specific Targeted Research Projects MANASP (LSHE-CT-2006), contract 037899 (FP6), and SYBARIS (LSHE-CT-2006), contract 242220 (FP7), and the Italian Projects PRIN 2007KLCKP8_004 (L.R.) and 2007XYB9T9_001 (S.B.). C.C. and A. Carvalho were supported by Fundação para a Ciência e Tecnologia, Portugal (fellowship contracts SFRH/BD/65962/2009 and SFRH/BPD/46292/2008, respectively).
Authorship
Contribution: C.C., J.-P.L., F.R., F.A., L.R., and A. Carvalho designed the study; C.C. and A. Carvalho obtained genetic data; M.D.I., A.P., L.P., F.F., A. Carotti, A.V., and F.A. obtained clinical data; C.C. performed statistical analyses; C.C. and K.P. performed the in vitro experiments with human cells; S.B., G.G., S.Z., T.Z., and C.D.A. performed the murine experiments; C.C., L.R., and A. Carvalho drafted the manuscript; and all the authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Agostinho Carvalho, Microbiology Section, Department of Experimental Medicine and Biochemical Sciences, University of Perugia, Via del Giochetto, 06126 Perugia, Italy; e-mail: aacarvalho2008@gmail.com.