To the editor:
The Janus kinase 2 V617F mutation (JAK2V617F) was the first common molecular marker for the characterization of Philadelphia chromosome–negative myeloproliferative neoplasms (Ph− MPN).1 Depending on the entity, 50% to 95% of Ph− MPN are JAK2V617F-positive.1,2 Over the past few years, several other molecular defects have been described but these aberrations are detectable only in a smaller subfraction of Ph− MPN cases (< 10%), for example, myeloproliferative leukemia virus oncogene (MPL), tet oncogene family member 2 (TET2) or additional sex combs like 1 (Drosophila) (ASXL1). However, approximately 50% of these cases are also JAK2V617F-positive,2,3 making these infrequent markers not useful for routine diagnostics.
Recently, mutations in the isocitrate dehydrogenase 1 (IDH1) and IDH2 have been reported in Ph− MPN, myelodysplastic neoplasms/syndromes (MDS) and acute myeloid leukemias.4-7 These mutant enzymes exhibit decreased affinity to isocitrate, which is decarboxylated to 2-oxoglutarate by the wild-type enzymes. Mutant IDH exhibits an aberrant catalytic activity toward a-ketoglutarate, which results indirectly in accumulation of 2-hydroxyglutarate and activation of hypoxia inducible factor 1 α subunit (HIF-1α).8 The IDH mutation and JAK2V617F can occur in parallel in one aberrant hematopoietic stem cell clone.5 In Ph− MPN, the mutation frequency is 0.8% for essential thrombocythemia (ET), 1.9% for polycythemia vera (PV), 4.2% for primary myelofibrosis (PMF),6 and 3.6% for MDS.7
The aim of our study was to evaluate the usefulness of IDH mutation analysis as an additional marker for routine molecular diagnostics of chronic stage myeloid neoplasms, particularly Ph− MPN and MDS.
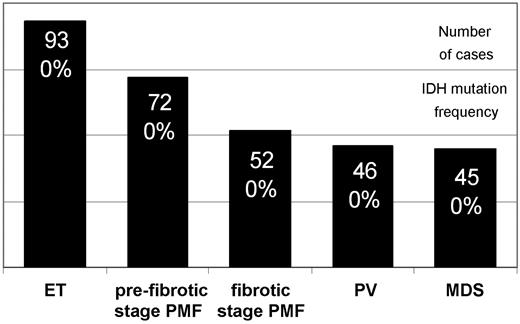
DNA samples (n = 326) from total bone marrow cells were analyzed with a Pyrosequencer assay (Biotage).2 A representative number of samples from ET (n = 93), prefibrotic stage PMF (n = 72), fibrotic stage PMF (n = 52), PV (n = 46), MDS (n = 45), and nonneoplastic controls (n = 18) were evaluated. Ph− MPN and MDS samples were derived from previously characterized cohorts (98% JAK2V617F PV, 48% JAK2V617F ET and PMF).2,9 Bone marrow samples were formalin-fixed and paraffin-embedded and were retrieved from the tissue archive of the Institute of Pathology (Hannover Medical School). The retrospective analysis had been approved by the local ethics committee.
None of the 263 Ph− MPN and 45 MDS cases showed detectable IDH mutations in codons R132 and R172 in IDH1 and IDH2, respectively (Figure 1). In Ph− MPN, JAK2V617F as well as IDHR132H can be acquired in a small subfraction of cells and can comprise a range of 5% to 10% of mutant alleles and < 5% of cells,10 respectively.2 Thus, we cannot exclude that some cases with a very low mutant allele burden or cases with other IDH aberrations were not detectable by this methodology.
IDH mutation analysis in bone marrow cells from myeloid neoplasms. The figure illustrates the large number of samples analyzed (black columns) but absence of IDH mutated cases in this cohort of Ph− MPN and MDS. DNA was extracted with the DNeasy kit (Qiagen). The following custom-made primer sets were used for mutant allele quantification at IDH1 codon R132 (forward 5′-biotin-gcttgtgagtggatgggtaaa, reverse 5′-gacttacttgatccccataagca, 65 bp; sequencing primer-1, 5′-tccccataagcatgac, and primer-2, 5′-gatccccataagcatg, covering the hotspot bases in codon R132) and IDH2 codon R172 (forward 5′-atcccacgcctagtccct, reverse 5′-biotin-tctccaccctggcctacc, 83 bp; sequencing primer 5′-ccatcaccattggca). Wild-type status of one control was confirmed by capillary sequencing: IDH1 (forward 5′-caagttgaaacaaatgtggaaatcac, reverse 5′-gtcacttggtgtgtaggttatctctctac, 223 bp) and IDH2 (forward 5′-tggaagagttcaagctgaagaagat, reverse 5′-tgccatcttttggggtgaag, 237 bp).
IDH mutation analysis in bone marrow cells from myeloid neoplasms. The figure illustrates the large number of samples analyzed (black columns) but absence of IDH mutated cases in this cohort of Ph− MPN and MDS. DNA was extracted with the DNeasy kit (Qiagen). The following custom-made primer sets were used for mutant allele quantification at IDH1 codon R132 (forward 5′-biotin-gcttgtgagtggatgggtaaa, reverse 5′-gacttacttgatccccataagca, 65 bp; sequencing primer-1, 5′-tccccataagcatgac, and primer-2, 5′-gatccccataagcatg, covering the hotspot bases in codon R132) and IDH2 codon R172 (forward 5′-atcccacgcctagtccct, reverse 5′-biotin-tctccaccctggcctacc, 83 bp; sequencing primer 5′-ccatcaccattggca). Wild-type status of one control was confirmed by capillary sequencing: IDH1 (forward 5′-caagttgaaacaaatgtggaaatcac, reverse 5′-gtcacttggtgtgtaggttatctctctac, 223 bp) and IDH2 (forward 5′-tggaagagttcaagctgaagaagat, reverse 5′-tgccatcttttggggtgaag, 237 bp).
A molecular marker such as JAK2V617F supports the diagnosis of a histomorphologically based Ph− MPN diagnosis and thus is very helpful in distinguishing reactive from neoplastic myeloproliferation. However, due to the low occurrence of mutations, the screening for IDH1 and IDH2 aberrations is not a useful tool for the routine diagnostic algorithm in chronic stage Ph− MPN and MDS.
Authorship
Acknowledgments: The authors thank Ms Sabine Schröter and Ms Nadine Preiss for their skillful help in the laboratory.
This work was supported by a research grant from Deutsche Forschungsgemeinschaft (BO 1954/1-2 to O.B. and H.K.) and by the BMBF (IFB-Tx to O.B.).
Contribution: K.H., B.M.E., and O.B. conducted molecular analyses; H.K. evaluated bone marrow histopathology; and all authors designed the study, interpreted data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kais Hussein, MD, Institute of Pathology, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: Hussein.Kais@MH-Hannover.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal