Abstract
Lysophosphatidic acid (LPA) is a potent lipid mediator with a wide variety of biological actions mediated through G protein-coupled receptors (LPA1-6). LPA4 has been identified as a G13 protein-coupled receptor, but its physiological role is unknown. Here we show that a subset of LPA4-deficient embryos did not survive gestation and displayed hemorrhages and/or edema in many organs at multiple embryonic stages. The blood vessels of bleeding LPA4-deficient embryos were often dilated. The recruitment of mural cells, namely smooth muscle cells and pericytes, was impaired. Consistently, Matrigel plug assays showed decreased mural cell coverage of endothelial cells in the neovessels of LPA4-deficient adult mice. In situ hybridization detected Lpa4 mRNA in the endothelium of some vasculatures. Similarly, the lymphatic vessels of edematous embryos were dilated. These results suggest that LPA4 regulates establishment of the structure and function of blood and lymphatic vessels during mouse embryogenesis. Considering the critical role of autotaxin (an enzyme involved in LPA production) and Gα13 in vascular development, we suggest that LPA4 provides a link between these 2 molecules.
Introduction
Lysophosphatidic acid (LPA; 1- or 2-acyl-sn-glycerol-3-phosphate) is a bioactive lipid molecule with a phosphate, a glycerol, and a fatty acid in its structure.1 The cellular effects of LPA include proliferation, migration, cytokine secretion, and morphological change.2 These pleiotropic actions allow LPA to participate in a wide variety of biological processes, such as brain development, oncogenesis, and wound healing.2 To date, at least 6 subtypes of LPA receptor have been identified. LPA1/Edg-2 (endothelial cell differentiation gene 2)/Vzg-1 (ventricular zone gene 1),3 LPA2/Edg-4,4 and LPA3/Edg-75 receptors are members of the Edg family and are 50%-60% homologous to each other. On the other hand, LPA4/GPR23/p2y9,6 LPA5/GPR92,7 and LPA6/p2y58 form a distinct family.9,10 Biochemical studies have revealed that each LPA receptor couples to multiple, but specific, G proteins including G12/13, Gi, Gq, and Gs, leading to activation of diverse signaling cascades that involve phosphoinositide 3-kinase, phospholipase C, mitogen-activated protein kinase, Rho family GTPase, and adenylyl cyclase.2
Insights into the biological functions of LPA receptors have been provided by knockout studies in mice. LPA1-deficient mice showed partial postnatal lethality due to a suckling defect resulting from impaired olfaction.11 Surviving LPA1-deficient mice were protected from pulmonary fibrosis.12 The absence of LPA2 attenuated tumorigenesis in the colon induced by azoxymethane and dextran sulfate.13 Deletion of the LPA3 receptor led to anomalous spacing and timing of blastocyst implantation, which was associated with reduced uterine expression of cyclooxygenase-2 mRNA.14 Although LPA4 was shown to mediate LPA-induced suppression of cell migration in vitro,15 the physiological role of LPA4 has not as yet been investigated.
In this report, we disrupted the LPA4-encoding gene in mouse embryonic stem (ES) cells and found that LPA4-deficient mice displayed abnormalities in the blood vascular systems, which caused lethality of embryos and neonates. Our studies revealed a novel role for LPA4 in the development of murine blood and lymphatic vessels.
Methods
Mouse breeding
Mice were housed under specific pathogen-free conditions in an air-conditioned room and fed standard laboratory chow ad libitum (MF; Oriental Yeast), in accordance with institutional guidelines. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Tokyo. During the mating of female mice (more than 8 weeks old), the presence of the vaginal plug, as evidence of copulation, was checked for every morning. Litter sizes were determined during the evening of the day of birth (P0). Pups were weaned at 3 weeks old (P21).
Generation of LPA4-deficient mice
LPA4-deficient mice were generated by standard gene targeting procedures using C57BL/6 ES cells.16 Briefly, the Lpa4 gene on the X chromosome was disrupted with a targeting vector (Figure 1A), which was designed to replace the Lpa4 open reading frame (ORF) in-frame with a LacZ-neo cassette. Genomic DNA samples from G418-resistant ES cells were digested with Eco81I and screened by Southern blot with the 5′ probe (shown in Figure 1A) to identify homologous recombinants (Figure 1B) as described below. To confirm the homologous recombination, the 3′ probe (shown in Figure 1A) was hybridized with TaqI-digested genomic DNA samples from the positive ES cell clones (Figure 1C). Of 281 G418-resistant ES cell clones screened, 3 were found to be disrupted for the Lpa4 gene by Southern blot analysis (Figure 1B-C). One clone was injected into C57BL/6 blastocysts. The resulting chimeric males were then bred to C57BL/6 females to produce F1 heterozygous (Lpa4+/-) female mice, which were then bred to generate LPA4-deficient mice. Mouse genotypes were determined by polymerase chain reaction (PCR) analysis using genomic DNA samples from the tails (Figure 1D), as described in PCR genotyping. To demonstrate that the expression of LPA4 was abolished in LPA4-deficient mice, Northern blot and reverse transcription-PCR (RT-PCR) analysis were performed using RNA samples extracted from whole embryos at embryonic day 12.5 (E12.5). LPA4 expression was observed in wild-type (WT), but not in LPA4-deficient mice (Figure 1E-F).
Generation of LPA4-deficient mice. (A) Strategy for homologous recombination at the mouse Lpa4 gene locus on the X chromosome. The entire ORF of LPA4 was replaced by the LacZ-neo cassette. The structure of the intact Lpa4 allele is shown at the top, the structure of the Lpa4 targeting vector is depicted in the middle, and the predicted structure of the targeted allele is shown at the bottom. (B) Southern blot analysis of genomic DNA from ES cells. Eco81I-digested genomic DNA samples from WT and LPA4-deficient ES cells were hybridized with the 5′ probe shown in panel A. Lane 1 is WT, while lanes 2, 3, and 4 represent LPA4-deficient cells. (C) Southern blot analysis of genomic DNA from ES cells. TaqI-digested genomic DNA samples from WT and LPA4-deficient ES cells were hybridized with the 3′ probe shown in panel A. Lane 1 is WT, while lanes 2, 3, and 4 represent LPA4-deficient cells. (D) PCR genotyping with genomic DNA from female tails. WT, wild-type; HT, heterozygous; KO, LPA4-deficient. (E) Northern blot analysis of Lpa4 mRNA expression. Each of total RNA samples (5 μg) from E12.5 whole embryos were hybridized with a probe containing the entire ORF of Lpa4 or a human β-actin cDNA probe. (F) RT-PCR with RNA samples from LPA4-deficient and WT mice. RNA was extracted from E12.5 whole embryos.
Generation of LPA4-deficient mice. (A) Strategy for homologous recombination at the mouse Lpa4 gene locus on the X chromosome. The entire ORF of LPA4 was replaced by the LacZ-neo cassette. The structure of the intact Lpa4 allele is shown at the top, the structure of the Lpa4 targeting vector is depicted in the middle, and the predicted structure of the targeted allele is shown at the bottom. (B) Southern blot analysis of genomic DNA from ES cells. Eco81I-digested genomic DNA samples from WT and LPA4-deficient ES cells were hybridized with the 5′ probe shown in panel A. Lane 1 is WT, while lanes 2, 3, and 4 represent LPA4-deficient cells. (C) Southern blot analysis of genomic DNA from ES cells. TaqI-digested genomic DNA samples from WT and LPA4-deficient ES cells were hybridized with the 3′ probe shown in panel A. Lane 1 is WT, while lanes 2, 3, and 4 represent LPA4-deficient cells. (D) PCR genotyping with genomic DNA from female tails. WT, wild-type; HT, heterozygous; KO, LPA4-deficient. (E) Northern blot analysis of Lpa4 mRNA expression. Each of total RNA samples (5 μg) from E12.5 whole embryos were hybridized with a probe containing the entire ORF of Lpa4 or a human β-actin cDNA probe. (F) RT-PCR with RNA samples from LPA4-deficient and WT mice. RNA was extracted from E12.5 whole embryos.
Southern blot analysis
After digestion with restriction enzymes, the genomic DNA samples were separated by electrophoresis on 0.7% agarose gels and transferred to nylon membranes. As described previously,17 the membranes were hybridized with a [α-32P]-deoxycytidine triphosphate-labeled 5′ or 3′ probe located outside the targeted region (Figure 1A).
Northern blot analysis
Macroscopic observation
Under anesthesia with urethane injected intraperitoneally (1.5 g/kg of body weight), embryos and placentas were surgically removed from pregnant females at E10.5, E12.5, E14.5, and E18.5. After weighing, embryos and placentas were photographed, fixed in 10% buffered formalin, and paraffin-embedded for hematoxylin and eosin (H&E) staining. Macroscopic observation of internal organs of embryos was performed at E18.5. Briefly, after fixation of E18.5 embryos in Bouin solution, the thoracic and abdominal organs were examined by the microdissection method.18
In situ hybridization
Embryos at E18.5 were fixed with Tissue Fixative (Genostaff), embedded in paraffin, and sectioned at 7 μm. After dewaxing, the tissue sections were fixed in 4% paraformaldehyde, treated with 8 μg/mL proteinase K, refixed in 4% paraformaldehyde, and placed in 0.2N HCl. Then the sections were acetylated by incubation with 0.25% acetic anhydride in 0.1M triethanolamine-HCl, pH 8.0, and dehydrated. A digoxigenin (DIG)-labeled cRNA probe, corresponding to nucleotides 250-714 of mouse Lpa4 (GenBank accession no. NM_175271), was generated by in vitro transcription using an RNA labeling kit (DIG RNA Labeling Mix; Roche). Hybridization was performed with 300 ng/mL probe in Probe Diluent-1 (Genostaff) at 60°C for 16 hours. After hybridization, the sections were washed in 5 × saline-sodium citrate (SSC) at 50°C for 20 minutes and then in 50% formamide and 2 × SSC at 50°C for 20 minutes, followed by RNase treatment with 50 μg/mL RNase A in 10mM tris(hydroxymethyl) aminomethane (Tris)-HCl, pH 8.0, 1M NaCl, and 1mM ethylenediaminetetraacetic acid. Then the sections were washed twice with 2 × SSC at 50°C for 20 minutes, twice with 0.2 × SSC at 50°C for 20 minutes, and once with 0.1% Tween-20 in Tris-buffered saline (TBST). After treatment with 0.5% blocking reagent (Roche) in TBST, the sections were incubated with anti-DIG alkaline phosphatase-conjugated antibody (Roche) diluted 1:1000 with TBST, washed twice with TBST, and incubated in 100mM NaCl, 50mM MgCl2, 0.1% Tween-20, and 100mM Tris-HCl, pH 9.5. Before mounting with Malinol (Mutoh Chemical), the color reaction and counterstaining were performed with nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolylphosphatase p-toluidine salt solution(Sigma-Aldrich) and Kernechtrot stain solution (Mutoh Chemical), respectively.
Immunohistochemical analysis
Immunohistochemical analyses of embryos at E18.5 were performed with the antibody for α-smooth muscle actin (α-SMA, a mural cell marker) or von Willebrand factor (VWF). Briefly, paraffin-embedded embryo sections were deparaffinized. Endogenous peroxidase activity was quenched by incubation in 3% hydrogen peroxide in phosphate-buffered saline for 10 minutes. Then, the sections were incubated with the primary antibody (a monoclonal mouse anti-human α-SMA antibody; DAKO Japan) or a polyclonal rabbit anti-human VWF (DAKO Japan), respectively, at room temperature for 10 minutes. As the secondary antibody, a Histofine MOUSESTAIN kit or Histofine Simple Stain Mouse MAX-PO (Nichirei Biosciences) was used, respectively. The color reaction was developed with 0.02% 3,3′-diaminobenzidine. Nuclei were counterstained with Mayer hematoxylin.
Immunohistochemical analyses of embryos at E14.5 and E18.5 were performed as described previously19 with minor modifications. For staining of lymphatic vessels, a rabbit polyclonal antibody to mouse lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1, a lymphatic endothelial cell marker, 1:200 dilution; Abcam) was used. A rat monoclonal antibody to mouse platelet/endothelial cell adhesion molecule 1 (PECAM1, an endothelial cell marker, 1:200 dilution; BD Pharmingen) was used for staining of blood vessels. Subsequently, specimens were incubated with a corresponding secondary antibody labeled with Alexa Fluor-594 (for the LYVE1 staining) or Alexa Fluor-488 (for the PECAM1 staining) (1:200 dilution; Invitrogen). Cell nuclei were counterstained with 20μM TOTO-3 (Invitrogen).
In vivo Matrigel plug assay
This assay was conducted according to a previously described method20,21 with minor modifications. Briefly, recombinant human vascular endothelial growth factor A (VEGFA, VEGF 165; R&D Systems), fibroblast growth factor 2 (R&D Systems), and heparin (Ajinomoto Pharma) were mixed with the regular Matrigel (BD Biosciences) at final concentrations of 200 ng/mL, 1 μg/mL, and 0.1 mg/mL, respectively. The Matrigel (500 μL each) was injected subcutaneously into bilateral abdominal regions of 9- or 10-week-old male mice. The Matrigel plugs were harvested on day 7. One side of the Matrigel plug was fixed in 10% formaldehyde and then paraffin-embedded for H&E staining. Another was directly frozen for immunohistochemistry. The vessels of H&E-stained samples were photographed. For staining of mural cells or vascular endothelial cells, a Cy3-conjugated murine monoclonal antibody for α-SMA (1:200 dilution; Sigma-Aldrich) or a rat monoclonal antibody for murine PECAM1 (1:200 dilution; BD Pharmingen) was used, respectively. For PECAM1 staining, an Alexa Fluor-488–conjugated anti-rat immunoglobulin G (1:200 dilution; Invitrogen) was used as the secondary antibody. Mural cell coverage of endothelial cells was quantified as the ratio of PECAM1+/α-SMA+ (double positive) areas to PECAM1+ areas. The ratio of a specimen was determined as the average value of 3-9 fields.
Statistical analysis
Data were expressed as the mean ± SEM and analyzed using GraphPad Prism 4 software (GraphPad Software). A value of P < .05 was considered statistically significant.
Results
High lethality rate of LPA4-deficient mice
At weaning on postnatal day (P) 21, the number of LPA4-deficient mice (Lpa4-/- females and Lpa4-/Y males) was approximately 30% less than the value expected from Mendelian ratios, while the number of heterozygous mice was not significantly reduced (Table 1). These data suggest that a subset of the LPA4-deficient mice either did not survive gestation or died before weaning. Further mating experiments revealed that the average litter sizes of Lpa4+/+ × Lpa4+/Y, Lpa4+/+ × Lpa4-/Y, Lpa4-/- × Lpa4+/Y, and Lpa4-/- × Lpa4-/Y were 7.8 ± 0.3 (n = 59), 7.7 ± 0.2 (n = 49), 6.2 ± 0.3 (n = 39), and 5.1 ± 0.3 (n = 47), respectively (Figure 2A). The latter 2 matings yielded significantly smaller litter sizes than the former 2 matings, which did not produce LPA4-deficient mice. Comparing the latter 2 matings, Lpa4-/- × Lpa4-/Y, in which all embryos were LPA4-deficient, yielded significantly smaller litter sizes than Lpa4-/- × Lpa4+/Y, where only male embryos were LPA4-deficient (Figure 2A). These results further support the possibility that LPA4-deficient embryos either did not survive gestation or died just after parturition. As expected, the number of apparently viable embryos from E12.5 to E18.5 was significantly lower in Lpa4−/− × Lpa4−/Y than in Lpa4+/+ × Lpa4+/Y (Figure 2B). Meanwhile, there was no significant difference in the number of implantation sites at E4.5 between Lpa4+/+ and Lpa4−/− pregnant females mated to Lpa4+/Y and Lpa4−/Y males, respectively (Figure 2B).
Number of offspring of each genotype at 3 weeks of age
| Parental genotypes . | Offspring genotype . | ||||
|---|---|---|---|---|---|
| Female . | Male . | ||||
| Female × Male | +/+ | +/− | −/− | +/Y | −/Y |
| +/+ × +/Y | 206 | − | − | 218 | − |
| −/− × +/Y | − | 110 | − | − | 75 |
| +/− × +/Y | 74 | 73 | − | 74 | 56 |
| +/− × −/Y | − | 87 | 60 | 82 | 55 |
| Parental genotypes . | Offspring genotype . | ||||
|---|---|---|---|---|---|
| Female . | Male . | ||||
| Female × Male | +/+ | +/− | −/− | +/Y | −/Y |
| +/+ × +/Y | 206 | − | − | 218 | − |
| −/− × +/Y | − | 110 | − | − | 75 |
| +/− × +/Y | 74 | 73 | − | 74 | 56 |
| +/− × −/Y | − | 87 | 60 | 82 | 55 |
Partial lethality of LPA4-deficient embryos and pups. (A) Litter sizes of various crosses. Each dot represents 1 litter. *P < .05, **P < .01, and ***P < .001, using 1-way analysis of variance (ANOVA) followed by Tukey post-hoc test. (B) Change in the number of apparently viable embryos from E4.5 to E18.5. Data represent the mean ± SEM. Numbers in parentheses represent the pregnant female studied. *P < .05 and **P < .001, using 2-way ANOVA followed by Bonferroni post-hoc test. (C) Number of dead pups by weaning at P21. Data represent the mean ± SEM. Numbers in parentheses represent the litter studied. *P < .05 and **P < .001, using ANOVA followed by Tukey post-hoc test. F, female; M, male.
Partial lethality of LPA4-deficient embryos and pups. (A) Litter sizes of various crosses. Each dot represents 1 litter. *P < .05, **P < .01, and ***P < .001, using 1-way analysis of variance (ANOVA) followed by Tukey post-hoc test. (B) Change in the number of apparently viable embryos from E4.5 to E18.5. Data represent the mean ± SEM. Numbers in parentheses represent the pregnant female studied. *P < .05 and **P < .001, using 2-way ANOVA followed by Bonferroni post-hoc test. (C) Number of dead pups by weaning at P21. Data represent the mean ± SEM. Numbers in parentheses represent the litter studied. *P < .05 and **P < .001, using ANOVA followed by Tukey post-hoc test. F, female; M, male.
We next examined Lpa4+/Y and Lpa4−/Y male littermates at E18.5 obtained by mating Lpa4+/− females with Lpa4+/Y males. No significant differences in the weight and histology of the placenta were observed between Lpa4+/Y and Lpa4−/Y embryos (data not shown). However, the number of Lpa4−/Y male embryos was decreased by 22.5% compared with that of Lpa4+/Y male embryos (40 vs 31). These results strongly suggest that the high fatality rate of LPA4-deficient embryos may be mainly due to the LPA4 deficiency in the embryos rather than that in the maternal contribution from the placenta.
Unlike LPA3-deficient mice,14 delayed implantation and abnormal embryo spacing were not observed in Lpa4−/− pregnant females at 4.5 and 5.5 days postcoitum, whose implantation sites were stained with Evans blue dye (data not shown).
An average of 1.8 ± 0.2 (approximately 35%) pups from Lpa4−/− × Lpa4−/Y died by the time of weaning (Figure 2C). This value was significantly higher than the average number of dead pups from Lpa4+/+ × Lpa4+/Y, Lpa4+/+ × Lpa4−/Y, or Lpa4−/− × Lpa4+/Y matings (Figure 2C and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). As was the case for litter size, LPA4 deficiency may be relevant to the death of pups before weaning.
Abnormalities in LPA4-deficient embryos during pregnancy
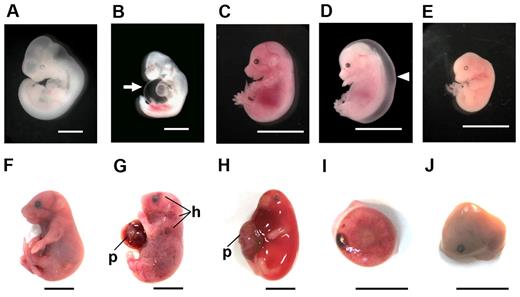
Various abnormalities were observed in LPA4-deficient embryos at each stage of gestation, as shown in Table 2. At E10.5, 72.0% of the LPA4-deficient embryos were apparently normal (Figure 3A), whereas some (14.0%) mutant embryos had effusions in the pericardial space or body surface (Figure 3B). Just over a third (35.9%) of the mutant embryos at E12.5 exhibited various developmental anomalies. At E14.5, 18.9% of mutant embryos suffered from subcutaneous edema and/or hemorrhage (Figure 3D), while only 54.0% of embryos were apparently normal (Figure 3C). Small and/or fragile mutant embryos were present at this embryonic stage (Figure 3E) as well as at E10.5 and E12.5. The proportion of grossly normal mutant embryos remained low (49.7%) at E18.5 (Figure 3F). In addition, a small but distinct proportion (10.5%) of the E18.5 mutant embryos showed subcutaneous severe edema and/or hemorrhage (Figure 3G-H). Hemorrhagic sites were present in the head, neck, limbs, or other regions along the body wall. In addition, resorbed embryos (22.7%), retained placentas (11.0%; Figure 3I), and macerated embryos (3.9%; Figure 3J) were also observed. Most of these fatal events during pregnancy occurred much more frequently in LPA4-deficient embryos than in WT embryos (Table 2).
Phenotypic abnormalities of LPA4-deficient embryos at E10.5, E12.5, E14.5, and E18.5
| E10.5 . | WT* . | KO* . |
|---|---|---|
| Mating | 6 | 5 |
| Total embryos | 53 (100)† | 50 (100) |
| Apparently normal embryos | 48 (90.6) | 36 (72.0) |
| Small and/or fragile embryos | 0 (0) | 2 (4.0) |
| Effusions and/or bleeding | 0 (0) | 7 (14.0) |
| Resorbed embryos | 5 (9.4) | 5 (10.0) |
| E12.5 | WT | KO |
| Mating | 12 | 9 |
| Total embryos | 106 (100) | 78 (100) |
| Apparently normal embryos | 95 (89.6) | 50 (64.1) |
| Small and/or fragile embryos | 0 (0) | 7 (9.0) |
| Retained placentas | 0 (0) | 10 (12.8) |
| Resorbed embryos | 11 (10.4) | 11 (14.1) |
| E14.5 | WT | KO |
| Mating | 17 | 14 |
| Total embryos | 147 (100) | 137 (100) |
| Apparently normal embryos | 133 (90.4) | 74 (54.0) |
| Edema and/or hemorrhage | 4 (2.8) | 26 (18.9) |
| Small and/or fragile embryos | 2 (1.4) | 4 (3.0) |
| Retained placentas | 0 (0) | 13 (9.5) |
| Resorbed embryos | 8 (5.4) | 20 (14.6) |
| E18.5 | WT | KO |
| Mating | 20 | 21 |
| Total embryos | 173 (100) | 181 (100) |
| Apparently normal embryos | 164 (94.8) | 90 (49.7) |
| Edema and/or hemorrhage | 0 (0) | 19 (10.5) |
| Agnathia or omphalocele | 0 (0) | 4 (2.2) |
| Macerated embryos | 0 (0) | 7 (3.9) |
| Retained placentas | 0 (0) | 20 (11.0) |
| Resorbed embryos | 9 (5.2) | 41 (22.7) |
| E10.5 . | WT* . | KO* . |
|---|---|---|
| Mating | 6 | 5 |
| Total embryos | 53 (100)† | 50 (100) |
| Apparently normal embryos | 48 (90.6) | 36 (72.0) |
| Small and/or fragile embryos | 0 (0) | 2 (4.0) |
| Effusions and/or bleeding | 0 (0) | 7 (14.0) |
| Resorbed embryos | 5 (9.4) | 5 (10.0) |
| E12.5 | WT | KO |
| Mating | 12 | 9 |
| Total embryos | 106 (100) | 78 (100) |
| Apparently normal embryos | 95 (89.6) | 50 (64.1) |
| Small and/or fragile embryos | 0 (0) | 7 (9.0) |
| Retained placentas | 0 (0) | 10 (12.8) |
| Resorbed embryos | 11 (10.4) | 11 (14.1) |
| E14.5 | WT | KO |
| Mating | 17 | 14 |
| Total embryos | 147 (100) | 137 (100) |
| Apparently normal embryos | 133 (90.4) | 74 (54.0) |
| Edema and/or hemorrhage | 4 (2.8) | 26 (18.9) |
| Small and/or fragile embryos | 2 (1.4) | 4 (3.0) |
| Retained placentas | 0 (0) | 13 (9.5) |
| Resorbed embryos | 8 (5.4) | 20 (14.6) |
| E18.5 | WT | KO |
| Mating | 20 | 21 |
| Total embryos | 173 (100) | 181 (100) |
| Apparently normal embryos | 164 (94.8) | 90 (49.7) |
| Edema and/or hemorrhage | 0 (0) | 19 (10.5) |
| Agnathia or omphalocele | 0 (0) | 4 (2.2) |
| Macerated embryos | 0 (0) | 7 (3.9) |
| Retained placentas | 0 (0) | 20 (11.0) |
| Resorbed embryos | 9 (5.2) | 41 (22.7) |
Wild-type (WT) and LPA4-deficient (KO) embryos were obtained from Lpa4+/+ × Lpa4+/Y and Lpa4−/− × Lpa4−/Y matings, respectively.
Percentages are given in parentheses.
Various embryonic abnormalities at different stages due to LPA4 deficiency. Mouse embryos at different developmental stages (E10.5, A-B; E14.5, C-E; E18.5, F-J) were macroscopically observed. Whereas many LPA4-deficient embryos appeared normal, like WT embryos (A,C,F), some mutant embryos had phenotypic abnormalities as follows: effusions in the pericardial space (arrow) at E10.5 (B), severe edema (arrowhead) (D), fragile embryo (E) at E14.5, subcutaneous hemorrhage (h) (G-H), edema (H), retained placenta (I), and macerated embryo (J) at E18.5. In panels G and H, embryos have placentas (p). Scale bars, 1 mm in panels A-B; 10 mm in panels C-J.
Various embryonic abnormalities at different stages due to LPA4 deficiency. Mouse embryos at different developmental stages (E10.5, A-B; E14.5, C-E; E18.5, F-J) were macroscopically observed. Whereas many LPA4-deficient embryos appeared normal, like WT embryos (A,C,F), some mutant embryos had phenotypic abnormalities as follows: effusions in the pericardial space (arrow) at E10.5 (B), severe edema (arrowhead) (D), fragile embryo (E) at E14.5, subcutaneous hemorrhage (h) (G-H), edema (H), retained placenta (I), and macerated embryo (J) at E18.5. In panels G and H, embryos have placentas (p). Scale bars, 1 mm in panels A-B; 10 mm in panels C-J.
Bleeding with dilated blood vessels and impaired mural cell coverage in LPA4-deficient mice
Among 17 LPA4-deficient embryos (including apparently normal embryos) alive at E18.5 from 3 pregnant mice, pericardial hemorrhage (14 of 17; Figure 4B), thymic hemorrhage (4 of 17), and lung hemorrhage (1 of 17) were observed. On the other hand, none of 14 WT embryos alive at E18.5 from 2 pregnant mice had such abnormalities (Figure 4A). Histological analysis of LPA4-deficient embryos with bleeding phenotypes at E18.5 revealed that there were many lesions with hemorrhage in the pericardial cavities (Figure 4D), periocular tissues (Figure 4E-F), salivary glands, and subcutaneous tissues (data not shown). Furthermore, ventral skin blood vessels in bleeding LPA4-deficient embryos were dilated compared with those in WT embryos (Figure 4G-H). Additionally, immunohistochemical analysis of α-SMA revealed that some of the dilated blood vessels in those LPA4-deficient embryos were poorly covered with α-SMA+ cells (Figure 4I-J).
Abnormal and dilated blood vessels with bleeding in LPA4-deficient embryos. (A-B) The hearts from WT (A) and LPA4-deficient (B) embryos at E18.5 were examined macroscopically. Pericardial hemorrhage was observed only in the LPA4-deficient embryos (B, white bold arrows). Note that a cut was made in the blood-stained pericardium in panel B. h, heart; l, lung; t, thymus. (C-D) H&E staining was performed on heart sections of WT (C) and LPA4-deficient (D) embryos at E18.5. In LPA4-deficient embryos, blood cells had leaked into the pericardial cavity (D, black bold arrows). a, atrium; p, pericardium; v, ventricle. Scale bars, 500 μm. (E-F) H&E staining was performed on periocular tissue sections of LPA4-deficient embryos at E18.5 (E). The framed square area in panel E is magnified in panel F. In LPA4-deficient embryos with subcutaneous hemorrhage, red blood cells had leaked into the mesenchyme from a blood vessel. o, oculus; b, blood vessel. Scale bars, 200 μm. (G-H) H&E staining was performed on ventral skin sections of WT embryos (G) and LPA4-deficient embryos with subcutaneous hemorrhage (H) (as shown in Figure 3G) at E18.5. Blood vessels containing red blood cells are indicated by arrows. The bleeding LPA4-deficient embryos (H) had dilated blood vessels compared with WT embryos (G). e, epidermis. Scale bars, 500 μm. (I and J) Immunohistochemical staining for α-SMA was performed on ventral skin sections of WT embryos (I) and LPA4-deficient embryos with subcutaneous hemorrhage (J) at E18.5. Blood vessels containing red blood cells are indicated by arrowheads. Most WT blood vessels were covered with α-SMA+ cells, while some of the dilated blood vessels (asterisks) in LPA4-deficient embryos were poorly covered with α-SMA+ cells. e, epidermis. Scale bars, 500 μm.
Abnormal and dilated blood vessels with bleeding in LPA4-deficient embryos. (A-B) The hearts from WT (A) and LPA4-deficient (B) embryos at E18.5 were examined macroscopically. Pericardial hemorrhage was observed only in the LPA4-deficient embryos (B, white bold arrows). Note that a cut was made in the blood-stained pericardium in panel B. h, heart; l, lung; t, thymus. (C-D) H&E staining was performed on heart sections of WT (C) and LPA4-deficient (D) embryos at E18.5. In LPA4-deficient embryos, blood cells had leaked into the pericardial cavity (D, black bold arrows). a, atrium; p, pericardium; v, ventricle. Scale bars, 500 μm. (E-F) H&E staining was performed on periocular tissue sections of LPA4-deficient embryos at E18.5 (E). The framed square area in panel E is magnified in panel F. In LPA4-deficient embryos with subcutaneous hemorrhage, red blood cells had leaked into the mesenchyme from a blood vessel. o, oculus; b, blood vessel. Scale bars, 200 μm. (G-H) H&E staining was performed on ventral skin sections of WT embryos (G) and LPA4-deficient embryos with subcutaneous hemorrhage (H) (as shown in Figure 3G) at E18.5. Blood vessels containing red blood cells are indicated by arrows. The bleeding LPA4-deficient embryos (H) had dilated blood vessels compared with WT embryos (G). e, epidermis. Scale bars, 500 μm. (I and J) Immunohistochemical staining for α-SMA was performed on ventral skin sections of WT embryos (I) and LPA4-deficient embryos with subcutaneous hemorrhage (J) at E18.5. Blood vessels containing red blood cells are indicated by arrowheads. Most WT blood vessels were covered with α-SMA+ cells, while some of the dilated blood vessels (asterisks) in LPA4-deficient embryos were poorly covered with α-SMA+ cells. e, epidermis. Scale bars, 500 μm.
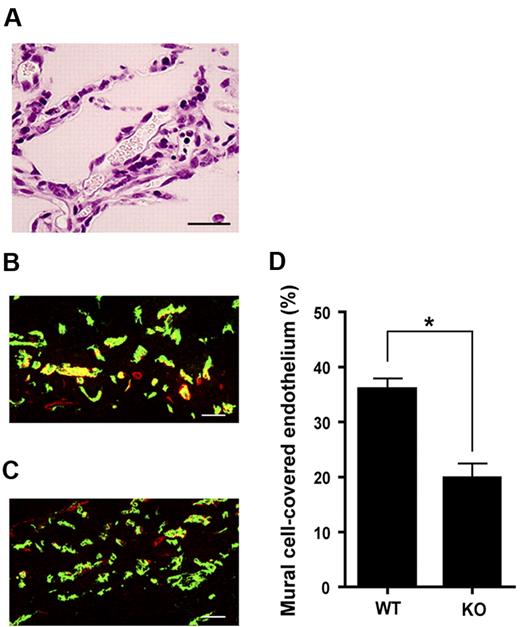
To examine further the involvement of LPA4 in vascular formation, we performed in vivo Matrigel plug assays. As shown in Figure 5A, vessel-like structures with red blood cells were formed in the plugs harvested from adult mice. Immunohistochemical analysis with antibodies to PECAM1 and α-SMA showed that the ratio of PECAM1+/α-SMA+ areas to PECAM1+ areas was significantly lower in LPA4-deficient mice than in WT mice (Figure 5B-D). Taken together with the bleeding phenotypes, these results suggest the importance of LPA4 in the development of blood vessels, especially in the coverage of endothelial cells by mural cells.
The importance of LPA4 in angiogenesis as evaluated by Matrigel plug assay. (A) An H&E-stained section prepared from a Matrigel plug. Erythrocytes are seen in a cavity that traces the shape of the endothelium-lined vessels. Scale bar, 50 μm. (B-C) Matrigel plug sections immunohistochemically stained for PECAM1 (green) and α-SMA (red). Representative staining of Matrigel plug sections obtained from WT (B) and KO (C) mice are shown. KO, LPA4-deficient. Scale bars, 50 μm. (D) Quantification of mural cell-covered endothelium. Data represent the mean ± SEM (n = 18 from 3 mice in each group). *P < .0001, using Mann-Whitney U test.
The importance of LPA4 in angiogenesis as evaluated by Matrigel plug assay. (A) An H&E-stained section prepared from a Matrigel plug. Erythrocytes are seen in a cavity that traces the shape of the endothelium-lined vessels. Scale bar, 50 μm. (B-C) Matrigel plug sections immunohistochemically stained for PECAM1 (green) and α-SMA (red). Representative staining of Matrigel plug sections obtained from WT (B) and KO (C) mice are shown. KO, LPA4-deficient. Scale bars, 50 μm. (D) Quantification of mural cell-covered endothelium. Data represent the mean ± SEM (n = 18 from 3 mice in each group). *P < .0001, using Mann-Whitney U test.
To assess potential defects in blood coagulation, another important cause of bleeding, a functional test of tail bleeding was performed. There was no significant difference in bleeding time between LPA4-deficient and WT mice (supplemental Figure 2A). The platelet numbers in the peripheral blood were normal in LPA4-deficient and WT adult mice (supplemental Figure 2B). Thus, the hemorrhagic diathesis is likely due to defects in blood vessels but not blood coagulation.
Dilation of the lymph sacs and lymphatic vessels in LPA4-deficient mice
At E11.5-E14.5, lymphatic endothelial precursors migrate centrifugally from the cardinal vein to form adjacent lymph sacs, the first lymphatic structures.22 At E14.5, 18.9% of LPA4-deficient embryos suffered from edema and/or hemorrhage as mentioned earlier. Abnormal lymphatic structure or function can be a cause of edema.23-25 Therefore, transverse sections of the cervix, including typical lymphatic sacs that surround the subclavian arteries, were immunostained with antibodies against PECAM1 and LYVE1 (Figure 6A-C). In both edematous LPA4-deficient and apparently normal LPA4-deficient embryos, significant dilation of the lymph sacs was observed compared with WT embryos (Figure 6D). Moreover, the lymph sacs of edematous LPA4-deficient emryos were significantly larger than those of apparently normal LPA4-deficient embryos (Figure 6D).
Abnormal and dilated lymphatic vessels with edema in LPA4-deficient embryos. (A-C) Transverse sections of the jugular area, including typical lymphatic sacs that surround the subclavian arteries, were immunostained with antibodies against PECAM1 (green), LYVE1 (red), and TOTO-3, a nuclear marker, (blue) in WT (A), apparently normal LPA4-deficient (B), and edematous LPA4-deficient (C) embryos at E14.5. SV, subclavian vein; SA, subclavian artery; CA, carotid artery; JV, jugular vein; LS, lymph sac. Scale bars, 100 μm. (D) Quantification of the area of lymph sacs in WT, apparently normal LPA4-deficient, and edematous LPA4-deficient embryos. Data represent the mean ± SEM (n = 13, 10, and 9, respectively). *P < .05 and **P < .001, using Kruskal-Wallis test followed by Dunn post-hoc test. KO, LPA4-deficient. (E-J) The ileum (E-G) and the skin (H-J) were immunostained with antibodies against PECAM1 (green), LYVE1 (red), and TOTO-3 (blue) in WT (E,H), apparently normal LPA4-deficient (F,I), and edematous LPA4-deficient (G,J) embryos at E18.5. Arrowheads, submucosal lymphatic vessels; arrows, subserosal lymphatic capillaries (lacteals); double arrowheads, severely dilated lymphatic vessels. Scale bars, 50 μm. (K-M) Quantification of area, number, and size ( = area/number) of the skin lymphatic vessels, respectively, in WT and apparently normal LPA4-deficient embryos. Data represent the mean ± SEM (n = 9 and 8, respectively). *P < .005 and **P < .0001, using unpaired 2-tailed t test.
Abnormal and dilated lymphatic vessels with edema in LPA4-deficient embryos. (A-C) Transverse sections of the jugular area, including typical lymphatic sacs that surround the subclavian arteries, were immunostained with antibodies against PECAM1 (green), LYVE1 (red), and TOTO-3, a nuclear marker, (blue) in WT (A), apparently normal LPA4-deficient (B), and edematous LPA4-deficient (C) embryos at E14.5. SV, subclavian vein; SA, subclavian artery; CA, carotid artery; JV, jugular vein; LS, lymph sac. Scale bars, 100 μm. (D) Quantification of the area of lymph sacs in WT, apparently normal LPA4-deficient, and edematous LPA4-deficient embryos. Data represent the mean ± SEM (n = 13, 10, and 9, respectively). *P < .05 and **P < .001, using Kruskal-Wallis test followed by Dunn post-hoc test. KO, LPA4-deficient. (E-J) The ileum (E-G) and the skin (H-J) were immunostained with antibodies against PECAM1 (green), LYVE1 (red), and TOTO-3 (blue) in WT (E,H), apparently normal LPA4-deficient (F,I), and edematous LPA4-deficient (G,J) embryos at E18.5. Arrowheads, submucosal lymphatic vessels; arrows, subserosal lymphatic capillaries (lacteals); double arrowheads, severely dilated lymphatic vessels. Scale bars, 50 μm. (K-M) Quantification of area, number, and size ( = area/number) of the skin lymphatic vessels, respectively, in WT and apparently normal LPA4-deficient embryos. Data represent the mean ± SEM (n = 9 and 8, respectively). *P < .005 and **P < .0001, using unpaired 2-tailed t test.
E18.5 mutant embryos (10.5%) had subcutaneous hemorrhage and/or edema (Table 2). Thus, we analyzed immunohistochemically the small intestine and skin of embryos at E18.5, which are highly sensitive to impairment of the lymphatic network formation.26,27 Using differential immunostaining for PECAM1 and LYVE1, we found several slightly enlarged submucosal lymphatic vessels in the small intestine of apparently normal LPA4-deficient embryos, compared with WT embryos (Figure 6E-F). Especially in LPA4-deficient edematous embryos, the subserosal lymphatic capillaries (the central lymphatic lacteals) were not detectable and many of the lymphatics of the submucosal plexus were severely enlarged (Figure 6G). In the intestinal villi of WT and apparently normal LPA4-deficient embryos, the subserosal lymphatic capillaries, which are the major sites of lipid uptake in the intestine, were surrounded by a basket of blood vessels (Figure 6E-F). However, the subserosal lymphatic capillaries of LPA4-deficient edematous embryos were frequently absent or abnormally short, whereas the subserosal blood vessels appeared normal (Figure 6G).
Also in the skin, LPA4-deficient apparently normal or edematous embryos had enlarged lymphatics, as compared with WT embryos (Figure 6H-J). Computer-assisted morphometric image analyses confirmed that the average size of dermal LYVE1+ lymphatic vessels was significantly increased in LPA4-deficient mice, whereas there was no difference in the number of lymphatic vessels (Figure 6K-M). No difference in the total area of blood vessels was found between WT and LPA4-deficient mice (data not shown). Furthermore, similar lymphatic vessel dilation was also observed in the lung of LPA4-deficient embryos (supplemental Figure 3).
A double-immunofluorescence analysis for the proliferation marker Ki-67 (a nuclear antigen) and the lymphatic marker prospero homeobox 1 (Prox1; a homeobox transcription factor) revealed proliferating lymphatic endothelial cells.28
In the head skin of embryos at E18.5, more than 20% of lymphatic endothelial cells were Ki-67+ in both LPA4-deficient and WT mice. There was no significant difference in the ratio of Ki-67+/Prox1+ cells to Prox1+ cells between LPA4-deficient and WT mice (supplemental Figure 4).
Expression of Lpa4 mRNA in some blood endothelial cells
In situ hybridization detected Lpa4 mRNA in the peripheral vasculatures of the tongue (Figure 7A), heart (Figure 7C arrow), muscle (Figure 7D), kidney (Figure 7G), lung, pancreas, and bowel (data not shown) in embryos at E18.5. However, large blood vessels did not express Lpa4 mRNA (Figure 7C arrowhead). To determine Lpa4 mRNA-expressing cells in the peripheral vasculatures, immunohistological analysis was performed with serial sections. Some of the Lpa4 mRNA-expressing cells stained positive for VWF (Figure 7G-H), which is commonly used as a marker for endothelial cells, but were negative for α-SMA (data not shown), a marker for mural cells. In E12.5 embryos, Lpa4 mRNA was detected in the vasculatures of the heart and lung (Figure 7E). Unlike E18.5 embryos, large blood vessels gave positive signals in these tissues. In addition, Lpa4 mRNA was detected in the nose, tongue, choroid plexus, and optic stalk (data not shown). In E10.5 WT embryos, Lpa4 mRNA was detected in the mesonephros and mandibular components of the first branchial arch as well as in blood vessels (data not shown). No positive signals were observed when the sense probe was used (Figure 7B,F).
Expression of Lpa4 mRNA in some blood vessels. (A-G) In situ hybridization of Lpa4 mRNA. The antisense probe was used in panels A,C-E,G. A positive signal was present in the peripheral vasculatures but not in the large blood vessels (C, upper right). In panels B,F, the sense probe was hybridized to serial sections of A and E, respectively, as negative controls. Little or no signal was observed. Nuclei were counterstained with Kernechtrot. (H) Immunohistochemistry for VWF counterstained with Mayer's hematoxylin. This was a serial section of panel G. Panels A-D,G-H were tissue sections from embryos at E18.5, while panels E-F were from embryos at E12.5. Lpa4 mRNA expression can be seen in the peripheral vasculatures of the tongue (A), heart (C), muscle (D), lung (E), and kidney (G). Scale bars, 200 μm in panels A-F and 50 μm in panels G,H.
Expression of Lpa4 mRNA in some blood vessels. (A-G) In situ hybridization of Lpa4 mRNA. The antisense probe was used in panels A,C-E,G. A positive signal was present in the peripheral vasculatures but not in the large blood vessels (C, upper right). In panels B,F, the sense probe was hybridized to serial sections of A and E, respectively, as negative controls. Little or no signal was observed. Nuclei were counterstained with Kernechtrot. (H) Immunohistochemistry for VWF counterstained with Mayer's hematoxylin. This was a serial section of panel G. Panels A-D,G-H were tissue sections from embryos at E18.5, while panels E-F were from embryos at E12.5. Lpa4 mRNA expression can be seen in the peripheral vasculatures of the tongue (A), heart (C), muscle (D), lung (E), and kidney (G). Scale bars, 200 μm in panels A-F and 50 μm in panels G,H.
Discussion
In 2003, we identified p2y9/GPR23 as LPA4 that is structurally distinct from the EDG family LPA receptors.6 In the present study, we disrupted the gene encoding LPA4 by homologous recombination in mice, to elucidate the functions of this receptor in vivo. The number of LPA4-deficient neonates was lower than that expected from Mendelian ratios. In LPA4-deficient embryos, we revealed several abnormalities in the blood and lymphatic vascular system.
In our examination of embryos at different gestational stages (E10.5 to E18.5), various degrees of subcutaneous hemorrhage were observed in a fraction of LPA4-deficient embryos, which suggests abnormalities in peripheral small blood vessels. Consistently, in situ hybridization detected Lpa4 mRNA in endothelial cells of the heart, kidney, lung, and other organs at E10.5, E12.5, and E18.5. In the LPA4-deficient embryos with subcutaneous hemorrhage, we found dilated blood vessels with decreased mural cell coating. The impaired mural cell coverage of endothelial cells was consistently observed in the Matrigel plug assay. Given the bleeding phenotypes and the impaired vascular formation, the LPA4 signaling pathway in the vascular endothelium seems to be important for vascular development, especially in mural cell recruitment to endothelial cells. Transforming growth factor-β (TGF-β)29-32 and platelet-derived growth factor-B (PDGF-B)33,34 signaling pathways have been shown to play important roles in vascular development. Indeed, pericardial effusion, enlarged blood vessels, and/or decreased mural cell recruitment were observed in various mutant mice deficient for signaling molecules of the TGF-β (TGF-β1,29 activin receptor-like kinase [ALK]-1,30 endoglin,31 and SMAD family member 5 [Smad5]32 ) and the PDGF-B (PDGF-B 33 and PDGF receptor β [PDGFRB]33,34 ) pathways (Table 3), suggesting that LPA4 affects these signaling pathways during vascular development.
Phenotypic similarities between mice deficient in LPA4 and other molecules
| Phenotypes of LPA4-deficient mice . | Molecules that cause similar phenotypes in mutants . | Reported stages . | Annotations . | References . |
|---|---|---|---|---|
| Abnormal blood vessels (hemorrhage) at E10.5-E18.5 | TGF-β1 | E9.5-E10.5 | g | 29 |
| ALK-1 | E9.5-E10.5 | a, e, f | 30 | |
| Endoglin | E9.5 | a, e, f | 31 | |
| Smad5 | E10.5-E11.5 | b, e, f | 32 | |
| ATX | E8.5-E10.5 | a, f | 37, 38 | |
| LPP3 | E9.5-E10.5 | f | 40 | |
| Endothelial Gα13 | E9.5-E11.5 | a, f | 41 | |
| PAR1 | E9.5-E10.5 | a, g | 43 | |
| VEGFR3 | E9.5 | a, f | 48 | |
| GPR4 | E10.5-postnatal | b, e, g | 50 | |
| S1P1 | E12.5-E14.5 | e, f | 47 | |
| S1P2/3 | E13.5-E15.5 | g | 51 | |
| PDGF-B | E17.5-E18.5 | b, e, f | 33 | |
| PDGFRB | E16-prebirth | b, e, f | 33, 34 | |
| Abnormal lymphatic vessels (edema) at E14.5-E18.5 | Prox1 | E14.5 | f | 23 |
| VEGFC | E15.5-E17.5 | f | 24 | |
| Foxc2 | E14.5-P1 | c, d, e, f | 25 | |
| T1α/podoplanin | postnatal | c, f | 26 | |
| Angiopoietin-2 | postnatal | c, e, g | 27 |
| Phenotypes of LPA4-deficient mice . | Molecules that cause similar phenotypes in mutants . | Reported stages . | Annotations . | References . |
|---|---|---|---|---|
| Abnormal blood vessels (hemorrhage) at E10.5-E18.5 | TGF-β1 | E9.5-E10.5 | g | 29 |
| ALK-1 | E9.5-E10.5 | a, e, f | 30 | |
| Endoglin | E9.5 | a, e, f | 31 | |
| Smad5 | E10.5-E11.5 | b, e, f | 32 | |
| ATX | E8.5-E10.5 | a, f | 37, 38 | |
| LPP3 | E9.5-E10.5 | f | 40 | |
| Endothelial Gα13 | E9.5-E11.5 | a, f | 41 | |
| PAR1 | E9.5-E10.5 | a, g | 43 | |
| VEGFR3 | E9.5 | a, f | 48 | |
| GPR4 | E10.5-postnatal | b, e, g | 50 | |
| S1P1 | E12.5-E14.5 | e, f | 47 | |
| S1P2/3 | E13.5-E15.5 | g | 51 | |
| PDGF-B | E17.5-E18.5 | b, e, f | 33 | |
| PDGFRB | E16-prebirth | b, e, f | 33, 34 | |
| Abnormal lymphatic vessels (edema) at E14.5-E18.5 | Prox1 | E14.5 | f | 23 |
| VEGFC | E15.5-E17.5 | f | 24 | |
| Foxc2 | E14.5-P1 | c, d, e, f | 25 | |
| T1α/podoplanin | postnatal | c, f | 26 | |
| Angiopoietin-2 | postnatal | c, e, g | 27 |
, pericardial effusion at E9.5-E10.5;
, dilation of blood vessels;
, dilation of lymphatic vessels;
, dilation of lymph sacs;
, abnormal coverage of mural cells;
, full lethality; and
, partial lethality.
Autotaxin (ATX) is an enzyme with lysophospholipase D activity, which converts lysophosphatidylcholine to LPA.35,36 Some ATX-deficient embryos at E10.5 had effusions in the pericardial space and/or the body surface.37,38 It is notable that similar effusions at this stage were observed in a fraction of LPA4-deficient embryos. To date, there has been no report that mentions effusions as a phenotype of LPA receptor–deficient mice. Because blood vessel formation or stabilization was impaired in ATX-deficient embryos, the ATX-LPA-LPA4 signaling axis may be involved in vascular development.
The lipid phosphate phosphatases (LPPs) regulate the biological activity and signaling of several bioactive phospholipids including LPA.39 LPA is converted to monoacylglycerol by the LPPs. LPP3-deficient mouse embryos died around E9.5 due to abnormal vasculogenesis.40 This report suggests that the control of LPA metabolism is also important for murine vascular development.
Gα13 activation in endothelial cells is known to play a key role during blood vessel development. LPA4-deficient embryos showed pericardial effusion at E10.5, which was also observed in vascular endothelium-specific Gα13-deficient embryos.41 In addition, we previously demonstrated that LPA4 couples to Gα13 in B103 rat neuroblastoma cells.42 Therefore, the loss of Gα13 activation by LPA4 in endothelial cells may account for the bleeding phenotypes in LPA4-deficient mice. These similarities in the phenotypes of LPA4-deficient embryos support the importance of LPA-LPA4-Gα13-Rho signaling in vascular development. Similarly, mice deficient for the Gα13-coupled protease-activated receptor 1 (PAR1) also showed partially penetrant embryonic lethality with bleeding from multiple sites.43 It is plausible that LPA4 cooperates with other Gα13-coupled G-protein-coupled receptors (GPCRs), including PAR1, in vascular development.
It has been reported that another lysophospholipid sphingosine 1-phosphate (S1P) regulates angiogenesis through S1P1 and S1P3.44 Especially, the Gi-coupled S1P1 has a major regulatory role in endothelial cell migration45 and stabilization of blood vessels by mural cells.46 As observed in LPA4-deficient embryos, S1P1-deficient mice exhibited embryonic hemorrhage with a reduction of mural cells adjacent to endothelial cells, leading to lethality between E12.5 and E14.5.47 Thus, both of these 2 lysophospholipids regulate vascular development by distinct intracellular signaling pathways.
In mice, lymphatic vessel development begins around E10. Many experimental data from mice support the hypothesis that lymphatic endothelial cells arise by sprouting from embryonic veins in the jugular and perimesonephric areas.22 At E11.5-E12.5, they migrate to form primary lymph sacs and the primary lymphatic plexus, which is composed of capillary-like vessels. After E12.5, lymphatic vessel differentiation and maturation occur, leading to synthesis of the main lymphatic vessel components. This process involves many factors such as Prox1, vascular endothelial growth factor C (VEGFC), vascular endothelial growth factor receptor 3 (VEGFR3), forkhead box C2 (Foxc2), T1α/podoplanin, and angiopoietin-2.22 In the present study, we found that a subset of LPA4-deficient embryos at E14.5 to E18.5 suffered from subcutaneous edema and/or hemorrhage.
In the area of jugular lymph sacs at E14.5, LPA4-deficient embryos had dilated lymph sacs as did Foxc2-deficient embryos at E14.5.25 Judging from our results and these previous reports, LPA4-deficient embryos might share the same mechanism for dilation of jugular lymph sacs as Foxc2-deficient embryos. All Foxc2-deficient neonates showed aortic arch anomalies, which were considered to be one of the causes of dilated jugular lymph sacs at E14.5.25 However, LPA4-deficient embryos at E18.5 had no cardiovascular phenotypes including aortic arch anomalies (data not shown). Thus, other unknown mechanisms may be responsible for the dilation of lymph sacs in LPA4-deficient embryos.
At E18.5, dilated lymphatic vessels in various sites including the skin and small intestine were still observed in a subset of LPA4-deficient embryos. These results indicated that LPA4 is also important for regulating lymphatic development and patterning in mice. As in T1α/podoplanin–deficient26 and angiopoietin-2–deficient27 mice, lacteals were not detectable and many of the lymphatics of the submucosal plexus were enlarged in the intestine of edematous LPA4-deficient mice at E18.5. Therefore, the enlargement of both dermal and submucosal intestinal lymphatics in LPA4-deficient mice is likely due to the lack of connecting lymphatics between the superficial and deep networks. Moreover, similar enlarged lymphatic vessels were seen in the skin of Foxc2-deficient embryos at E17.5.25 Notably, in these embryos, uneven lumen size and abnormal recruitment of mural cells were observed in collecting lymphatic vessels. Therefore, LPA4 might be involved in the recruitment of mural cells to lymphatic endothelial cells, as blood endothelial cells.
The phenotypes of abnormal blood or lymphatic vessels in LPA4-deficient embryos were shared by VEGFC-,24 VEGFR3-,48 and Foxc2-deficient25 embryos (Table 3). It was suggested that Foxc2 and VEGFR3 cooperate in lymphatic vascular patterning and that Foxc2 acts downstream of VEGFR3.25 Interestingly, a recent report demonstrated that LPA induces mRNA expression of VEGFC, one of the ligands for VEGFR3 in human umbilical vein endothelial cells.49 However, our quantitative PCR experiments with the embryonic small intestines, which are rich in lymphatic vessels, revealed no significant difference in the mRNA expression level of VEGFC, VEGFR3, or Foxc2 between WT and LPA4-deficient mice at E18.5 (data not shown), suggesting that LPA4 has little or no modulatory effect on the mRNA expression of these molecules involved in lymphatic vascular development. The LPA-LPA4 signaling may affect lymphatic vascular development by interacting the VEGFC-VEGFR3-Foxc2 signaling.
The precise molecular mechanisms underlying the role of LPA4 in lymphatic vessel formation remain to be elucidated. It is not clear if LPA directly affects lymphatic vessels through LPA4, because we could not detect the expression of Lpa4 mRNA in lymphatic vessels by in situ hybridization. At present, there are no established procedures for the successful isolation and expansion of primary murine lymphatic endothelial cells in vitro. In primary human dermal microvascular lymphatic endothelial cells and human lung microvascular lymphatic endothelial cells, Lpa4 mRNA expression was detected by RT-PCR (data not shown). These results suggest a direct role for LPA in lymphatic vessels at least in humans.
The partially penetrant lethality and hemorrhage in LPA4-deficient mice is reminiscent of the phenotypes in mice deficient for TGF-β1,29 GPR4,50 PAR1,43 and S1P2/351 (Table 3). Similar to GPR4-deficient mice and S1P2/3-deficient mice, surviving LPA4-deficient adult mice did not show obvious abnormalities under standard housing conditions. However, because a significantly higher number of LPA4-deficient pups than WT pups died before weaning, LPA4 seems to be involved in postnatal development as well as in embryonic development. Although the cause of partial embryonic and neonatal lethality remains to be resolved, compensatory mechanisms from other LPA receptors, other GPCRs and/or other pathways may be present during development.
Fang and colleagues recently reported LPA4-deficient mice with a mixed 129/Sv and C57BL/6 genetic background, but no abnormalities in the blood or lymphatic vessels were mentioned.15 Because our LPA4-deficient mice were on a pure C57BL/6 background, this apparent discrepancy may be caused by strain differences and/or different experimental conditions. It is of note that LPA4 deficiency led to the enhancement of LPA-induced Akt phosphorylation at Thr-308 in mouse embryonic fibroblasts (data not shown), as shown in the recent report.15
In the present study, we demonstrated that LPA4-deficient embryos exhibit abnormalities in both the blood and the lymphatic vessels. Although the detailed molecular mechanisms remain to be elucidated, our study clearly showed a novel role for this GPCR in the assembly and function of the blood and lymphatic vessels during mouse embryogenesis. In adults, LPA4 may be involved in tumor progression and inherited blood/lymphatic vessel diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof H. Kurihara and Dr K. Nishiyama (Department of Physiological Chemistry and Metabolism, Graduate School of Medicine, The University of Tokyo) for their helpful comments and discussions.
This work was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (to T.S. and S.I.), the Global COE Program (The University of Tokyo) from the Japan Society for Promotion of Sciences (to T.S.), a Health and Labor Sciences Research Grant for the Research on Allergic Disease and Immunology from the Ministry of Health, Labor, and Welfare of Japan (to S.I.), a grant to the Respiratory Failure Research Group from the Ministry of Health, Labor, and Welfare of Japan (to S.I.), and a grant from the Takeda Science Foundation (to S.I.).
Prof Kunihiko Tamaki died suddenly on July 24. We dedicate this paper in his memory.
Authorship
Contributions: H.S. and S.I. designed research, performed experiments, analyzed data, and wrote the paper; K.N., Y.K., M.A., K.Y., and F.H. performed experiments; S.S. and K.T. discussed the data; Y.M., M.R.K., and C.I. designed research, performed experiments, and analyzed data; K.S. provided ES cells and designed research; and K.M. and T.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Satoshi Ishii is the Department of Immunology, Graduate School of Medicine, Akita University, Akita, Japan.
Correspondence: Satoshi Ishii, Department of Immunology, Graduate School of Medicine, Akita University, 1-1-1 Hondo, Akita, Akita 010-8543, Japan; e-mail: satishii@med.akita-u.ac.jp.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal