Abstract
Inhibition of antigen-dependent B-cell receptor (BCR) signaling is considered a promising therapeutic approach in chronic lymphocytic leukemia (CLL), but experimental in vivo evidence to support this view is still lacking. We have now investigated whether inhibition of BCR signaling with the selective Syk inhibitor fostamatinib disodium (R788) will affect the growth of the leukemias that develop in the Eμ-TCL1 transgenic mouse model of CLL. Similarly to human CLL, these leukemias express stereotyped BCRs that react with autoantigens exposed on the surface of senescent or apoptotic cells, suggesting that they are antigen driven. We show that R788 effectively inhibits BCR signaling in vivo, resulting in reduced proliferation and survival of the malignant B cells and significantly prolonged survival of the treated animals. The growth-inhibitory effect of R788 occurs despite the relatively modest cytotoxic effect in vitro and is independent of basal Syk activity, suggesting that R788 functions primarily by inhibiting antigen-dependent BCR signals. Importantly, the effect of R788 was found to be selective for the malignant clones, as no disturbance in the production of normal B lymphocytes was observed. Collectively, these data provide further rationale for clinical trials with R788 in CLL and establish the BCR-signaling pathway as an important therapeutic target in this disease.
Introduction
The proliferation and survival of chronic lymphocytic leukemia (CLL) B cells is regulated by intracellular signaling pathways that are activated by various stimuli from the microenvironment. Among these, antigenic stimuli that are propagated through the B-cell receptor (BCR) are considered to play a key role in the initiation, maintenance, and evolution of the malignant clone.1-3
A role for antigen stimulation and selection in the pathogenesis of CLL was postulated many years ago, based on observations that the leukemic cells express a highly restricted immunoglobulin heavy-chain variable-region (IgVH) gene repertoire.4 This hypothesis was subsequently reinforced with the identification of CLL subsets with nearly identical (ie, stereotyped) B-cell receptors (BCRs), suggesting that the malignant clones are selected by sets of structurally similar antigens.5 The possibility that antigen stimulation may also be involved in disease progression is supported by the strong association between the clinical course in CLL and certain BCR-related features, such as IgVH gene mutation status and expression of the protein tyrosine kinase zeta-associated protein-70 (ZAP-70).6-8 In addition, immunophenotyping and gene-expression profiling studies have shown that CLL cells display features of lymphocytes that are continuously triggered by antigen in vivo. This especially refers to leukemic B cells from the poor prognosis subset, which more often exhibit a surface phenotype of recently activated B lymphocytes and show increased expression of genes that are induced in normal B cells by BCR engagement.9,10
The evidence that BCR signals may be involved in the initiation and evolution of CLL raised the possibility that inhibition of this pathway could be a therapeutically effective strategy in CLL. Previous studies by our group showed that sustained engagement of the BCR induces a powerful antiapoptotic program in CLL cells, which is characterized by the induction of several apoptosis regulatory proteins, including Mcl-1, Bcl-xL, and XIAP.11,12 Transduction of the prosurvival BCR signal was shown to involve the recruitment and activation of several kinases, including Lyn, Syk, Akt, and extracellular signal–related kinase (ERK). More recently, we showed that this antiapoptotic BCR signal can be completely abrogated with the selective small-molecule Syk inhibitor R406, suggesting that the Syk kinase could be a potential target for therapeutic intervention.13
In addition to blocking the antiapoptotic BCR signal, we observed that R406 is also moderately cytotoxic for unstimulated CLL cells.13 This effect of R406 was related to inhibition of the increased basal activity of Syk, a phenomenon that was recently described in CLL and several other B-cell malignancies and shown to contribute to the increased apoptosis resistance of the malignant lymphocytes.13-20 The reason for the increased basal Syk activity in CLL B cells is unclear at present, but could include persistent engagement of the BCR with autoantigen or exaggerated tonic BCR signaling due to mutations in regulatory BCR-signaling components.21-23
The previous studies with R406 suggested that this compound could be even more active in vivo, where both antigen-dependent and constitutive BCR signals should cooperate to increase the survival and proliferation of the malignant B cells. To test this possibility, we have now evaluated the efficacy of R406 and its prodrug fostamatinib disodium (also called R788) in the Eμ-T-cell leukemia 1 (TCL1) transgenic mouse model of CLL.24 Aged Eμ-TCL1 mice develop CD5+ B-cell leukemias that, like aggressive human CLL, show features of an antigen-driven process, including expression of stereotyped BCRs and reactivity with common autoantigens and microbial agents.25 A particularly common reactivity of the TCL1 leukemias is against phosphatidylcholine (PtC), an autoantigen that is exposed on the surface of senescent erythrocytes and apoptotic cells. B cells with this specificity express BCRs that are encoded by 1 of 2 VH/Vκ combinations (VH11/Vκ9 or VH12/Vκ4/5H) and have been shown in numerous studies to be positively selected and expanded by antigen.26-29 Thus, TCL1 leukemias reactive with PtC should represent an excellent model to study the activity of compounds that target the BCR-signaling pathway.
In the present study we show that treatment with R788 selectively inhibits the growth of TCL1 leukemias in vivo, without affecting the production of normal B lymphocytes. Importantly, we show that R788 is effective against TCL1 leukemias with anti-PtC specificity, suggesting that R788 functions, at least in part, by inhibiting antigen-dependent BCR signaling. These data further underscore the relevance of the BCR signaling pathway as a potential therapeutic target in CLL and suggest that CLL should be a particularly appropriate setting for future clinical trials with R788 and other Syk inhibitors.
Methods
Mouse models, TCL1 leukemia lines, and adoptive cell transfer experiments
Eμ-TCL1 transgenic mice, kindly provided by Dr Carlo Croce (Ohio State University, Columbus, OH), were backcrossed for 7 generations on a C57BL/6 background. Mice were housed under conventional barrier protection and monitored for the development of CLL-like disease by monthly white blood cell (WBC) counts using a Hemavet HV950FS hematology analyzer (Drew Scientific) and flow cytometric analysis. Mice were considered to have developed leukemic disease in the presence of at least 20% monoclonal CD5+ B cells in the peripheral blood and rising lymphocyte counts with respect to previous analyses. Overt leukemia was defined as at least 50% monoclonal CD5+ B cells in the peripheral blood and WBC above normal range (> 10.7 × 106/mL).
The TCL1 leukemia lines that were used in the adoptive-transfer experiments had been established from the original Eμ-TCL1 transgenic colony, which had a B6/C3H background. For this reason, the adoptive-transfer experiments with these leukemias were done in B6/C3H F1 recipients (6- to 8-week-old female mice; Harlan Laboratories BV). For adoptive transfer, 1.5 × 107 TCL1 leukemia cells were thawed, resuspended in 500 μL of phosphate-buffered saline (PBS), and injected intraperitoneally into the syngeneic recipients. Mice were followed for leukemia development as described above and were euthanized when they developed signs and symptoms of a moribund state, such as lethargy, aversion to activity, lack of sustained purposeful response to gentle stimuli, shallow or labored breathing, and other disabling symptoms. All animal procedures were performed in accordance with Italian national (Italian legislative decree 116/92 and European directive 8/609) and International Centre for Genetic Engineering and Biotechnology (ICGEB) institutional guidelines.
Purification, immunophenotyping, and culture of TCL1 leukemias and normal B cells
Mononuclear cells were separated from the spleens of normal or leukemic mice by Ficoll gradient centrifugation (Amersham Biosciences). Normal B cells were purified by negative selection using the EasySep mouse B-cell enrichment kit or positive selection using the EasySep mouse CD19 selection kit (both from StemCell Technologies). The purity of the selected populations was evaluated by staining with anti-CD5 phycoerythrin (PE)–conjugated and anti-B220 fluorescein isothiocyanate (FITC)–conjugated antibodies (BD Biosciences), followed by flow cytometric analysis on a FACSCalibur flow cytometer (BD Biosciences). The purity of the positively selected samples always exceeded 96%, whereas the purity of the negatively selected samples ranged from 82% to 87%. TCL1 leukemia cells were usually not purified because they represented more than 90% of the mononuclear cell population isolated from the spleens of leukemic animals. However, for analysis of ZAP-70 expression by Western blotting, contaminating T cells were eliminated with biotin-conjugated anti-mouse CD3 antibody (BD Biosciences) and EasySep magnetic particles (StemCell Technologies). Additional immunophenotyping of TCL1 leukemias was performed with antibodies specific for murine CD19, immunoglobulin M (IgM), and CD38 (BD Biosciences).
For the in vitro experiments, freshly isolated normal and leukemic cells were placed immediately in culture with RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2mM l-glutamine, and 1mM sodium pyruvate (Invitrogen), at 37°C in the presence of 5% CO2. BCR stimulations were performed on 1 × 107 cells/mL with 20 μg/mL goat F(ab′)2 anti–mouse IgM (Southern Biotechnology Associates) at 37°C for 3 minutes. The Syk inhibitor R406 (kindly provided by Rigel Pharmaceuticals) was used as indicated in the figures. R406 was prepared as described elsewhere.13 The R406 prodrug R788 was prepared as a 4-mg/mL solution in 0.1% carboxymethylcellulose sodium, 0.1% methylparaben, and 0.02% propylparaben (pH 6.5). The same vehicle without R788 was administered to the animals in the control groups.
Assessment of tumor burden
The spleen and 2 pairs of lymph nodes (inguinal and axillary) were collected from each mouse to assess the effect of R788 treatment on tumor burden. Single-cell suspensions were prepared from all samples and counted on a Hemavet HV950FS hematology analyzer. Red blood cells (RBCs) were lysed using RBC lysis buffer (155mM NH4Cl, 10mM KHCO3).
IgVH gene-sequence analysis
Polymerase chain reaction (PCR) amplification and sequencing of IgVH region genes were done as described elsewhere.25 Briefly, RNA was reverse transcribed using random hexamers and then PCR amplified with consensus FR1 family-specific primers in combination with a Cμ reverse primer. PCR products were purified with the QIAquick PCR purification kit (QIAGEN) and directly sequenced with the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems). Candidate germline genes were assigned by searching the IMGT/V-QUEST directory. The monoclonal nature of the TCL1 leukemias was confirmed by gene-scan fragment analysis of reverse transcription (RT)–PCR reactions that were performed with the same consensus FR1 family-specific primers and a tetrachloro-6-carboxy-fluorescein (TET)–labeled Cμ reverse primer. The PCR products were separated and visualized on an ABI 3100 genetic analyzer (Applied Biosystems).
Immunoblotting analysis
Cell pellets were lysed in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (10mM Tris-HCl (pH 7.4) 5mM EDTA [ethylenediamine tetraacetic acid], 150mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate) containing a 1:30 dilution of protease and a 1:75 dilution of phosphatase inhibitor cocktail for mammalian cells (Sigma-Aldrich). Proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on Immobilon-P polyvinylidene difluoride membranes (Millipore). Membranes were blotted at 4°C with the following antibodies: phospho-SykY352, phospho-SykYY525/6, phospho-ERKT202/Y204, phospho-AktS473, phospho-GSK3α/βS21/9, phospho-FoxO1T24/FoxO3aT32, B-cell linker protein (BLNK), ERK, Syk, poly(ADP-ribose)polymerase (PARP), Bim, ZAP-70, rabbit goat immunoglobulin G–horseradish peroxidase (IgG-HRP), mouse IgG HRP–linked (Cell Signaling Technology), phospho-BLNKY84 (BD Biosciences), Mcl-1 (Santa Cruz Biotechnology), and beta-actin (Sigma-Aldrich). Immunodetection was done with the enhanced chemiluminescence (ECL) Plus detection system (Amersham Biosciences) and the Gel Logic 2200 Imaging System (Eastman Kodak).
Apoptosis and proliferation assays
To evaluate the cytotoxic effect of R406 in vitro, TCL1 leukemia cells (1 × 106/200 μL) were placed in culture with increasing concentrations of R406. The percentage of apoptotic cells was determined by double staining with propidium iodide (PI) and annexin-A5–FITC conjugate (Nexins Research), according to the manufacturer's instructions. Ki-67 staining was performed with the FITC mouse anti–Ki-67 set, according to the instructions of the manufacturer (BD Pharmingen). Samples were analyzed on a FACSCalibur flow cytometer with CellQuest Version 3.3 software (BD Biosciences).
To evaluate apoptosis induced by R788 in vivo, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed on paraffin-embedded spleen sections with the In Situ Cell Death Detection kit (Roche Applied Science), according to the instructions of the manufacturer. TUNEL-positive cells were counted with National Institutes of Health ImageJ Version 1.43 software with the commands “density slicing” and “count particles” on binary images. The accuracy and appropriate cut-off level were determined by comparing counted images to the original.
In vivo 5-bromo-2-deoxyuridine (BrdU) labeling was done in animals with adoptively transferred leukemia that were treated for 7 days with vehicle control or R788. On the last day of treatment, each mouse received 20 mg/mL BrdU (BD Biosciences), in 2 divided doses during a 3-hour interval. Spleen samples were collected 6 hours after the second administration of BrdU. Frozen spleen sections were stained with the BrdU In-Situ Detection kit (BD Biosciences), according to the instructions of the manufacturer. BrdU positive cells were counted as described for the TUNEL assay.
Statistical analysis
Student t tests and Mann-Whitney rank-sum tests were performed to determine the significance of the differences between mean and median values, as appropriate. The survival curves and medians were calculated within subgroups with the method of Kaplan-Meier. The log-rank test was used to compare differences between estimated survival curves. All statistical analyses were performed using the SigmaStat 3.1 program (Systat Software). P values are indicated in the figures.
Results
R406 inhibits ligand-dependent and -independent BCR signaling in TCL1 leukemias in vitro
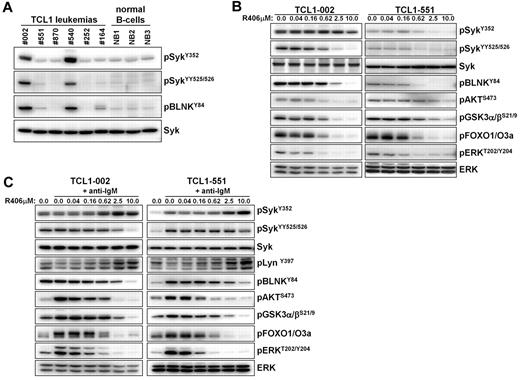
We started our study by investigating whether TCL1 leukemias express constitutively active Syk, as previously described for human CLL. Significant phosphorylation of Syk at the activating Y352 and YY525/526 residues was observed in 2 of the 6 investigated leukemias (TCL1-002 and TCL-540; Figure 1A). Phosphorylation of Syk correlated with phosphorylation of its direct substrate BLNK, further suggesting that Syk is constitutively active. In the remaining 4 leukemias, the intensity of the Syk signal was similar to the signal in normal splenic B cells. However, incubation with the Syk inhibitor R406 resulted in reduced basal phosphorylation of BLNK, Akt, glycogen synthase kinase-3 (GSK3), forkhead box O (FOXO) and ERK not only in cells with high (TCL-002) but also in cells with low levels of phosphorylated Syk (TCL1–551), suggesting that variable amounts of active Syk are present in all TCL1 leukemias (Figure 1B).
Analysis of Syk activity and BCR signaling in TCL1 leukemias in vitro. (A) The presence of active Syk protein was investigated by immunoblotting analysis with phospho-specific antibodies on cellular extracts prepared from spleens of mice with TCL1 leukemias. Normal B cells that were purified by negative selection from spleens of wild-type mice were used as controls. (B) Freshly isolated TCL1 leukemia cells were cultured for 90 minutes in the presence of the indicated concentrations of R406. The effect on the basal activity of signaling molecules downstream of Syk was investigated by immunoblotting analysis using phospho-specific antibodies. Syk and ERK served as loading controls. (C) TCL1 leukemia cells were preincubated for 90 minutes with the indicated concentrations of R406 prior to stimulation with anti-IgM antibody for 3 minutes. Cells were collected and analyzed as above.
Analysis of Syk activity and BCR signaling in TCL1 leukemias in vitro. (A) The presence of active Syk protein was investigated by immunoblotting analysis with phospho-specific antibodies on cellular extracts prepared from spleens of mice with TCL1 leukemias. Normal B cells that were purified by negative selection from spleens of wild-type mice were used as controls. (B) Freshly isolated TCL1 leukemia cells were cultured for 90 minutes in the presence of the indicated concentrations of R406. The effect on the basal activity of signaling molecules downstream of Syk was investigated by immunoblotting analysis using phospho-specific antibodies. Syk and ERK served as loading controls. (C) TCL1 leukemia cells were preincubated for 90 minutes with the indicated concentrations of R406 prior to stimulation with anti-IgM antibody for 3 minutes. Cells were collected and analyzed as above.
BCR engagement with anti-IgM antibody substantially increased the phosphorylation of several downstream signaling molecules, such as Akt, GSK3, FOXO, and ERK in the malignant B cells. This was observed both in leukemic cells with low and high levels of active Syk (TCL1-551 and TCL1-002, respectively; Figure 1C). However, an increase in the phosphorylation of Syk and its direct substrate BLNK was observed only in TCL1-551 cells, whereas no such changes were evident in TCL1-002 cells. A possible explanation for the absence of additional Syk and BLNK phosphorylation in TCL1-002 cells, despite efficient downstream signaling, is that most Syk molecules in these cells are already in an enzymatically active conformation, but remain outside of signaling complexes and become recruited to the signalosome only after acute BCR engagement. Importantly, R406 completely inhibited the anti-IgM induced BCR signal in both cell lines, which was evidenced by the inhibition of both proximal (eg, phosphorylation of SykYY525/526, and BLNKY84) and distal (eg, phosphorylation of Akt, GSK3, FOXO, and ERK) signaling events (Figure 1C). Interestingly, an increase in the amount of Syk phosphorylated at Y352 was observed in these experiments at higher R406 concentrations. Since this site in Syk is phosphorylated by Lyn, the most likely explanation for this finding is that Syk is involved in a negative regulatory circuit that inactivates Lyn after BCR engagement. Consistent with this possibility, we observed that the phosphorylation of Lyn at the activating Y397 residue increases in anti-IgM–stimulated cells when Syk is inhibited by R406.
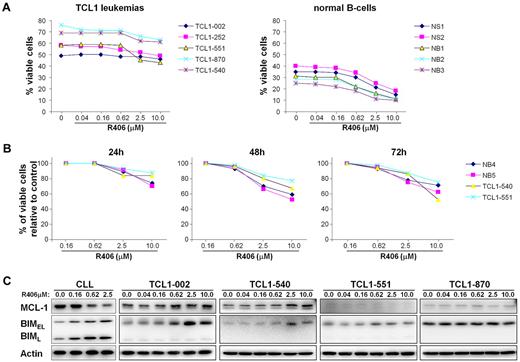
Since R406 has been shown to induce apoptosis in unstimulated human CLL cells, we next investigated whether it would have the same effect on TCL1 leukemias in vitro.13 As shown in Figure 2, a modest decrease in the percentage of viable (annexin V/PI negative) cells was observed at the highest R406 concentrations. However, a similar rate of apoptosis was observed with normal mouse B cells, suggesting that R406 is not selectively cytotoxic to the leukemic cells, despite the higher levels of constitutively active Syk. This was especially evident with the leukemic line TCL1-002, which expressed high levels of phospho-Syk, but appeared most resistant to the cytotoxic effect of R406.
Sensitivity of TCL1 leukemias to R406-induced apoptosis in vitro. (A) TCL1 leukemia cells were cultured for 48 hours with increasing concentrations of R406. The rate of apoptosis was determined by Annexin V/PI staining. Samples NB1, NB2, and NB3 are normal B cells that were purified by negative selection from the spleens of wild-type mice; samples NS1 and NS2 are mononuclear cells obtained from the spleens of the same animals. (B) Time-course analysis of in vitro sensitivity to R406. The percentage of viable cells relative to control (no R406) was determined in 2 TCL1 leukemias (TCL1-540 and TCL1-551) and 2 normal B-cell samples (NB4 and NB5) after 24, 48, and 72 hours incubation with the indicated concentrations of R406. (C) Human CLL cells and TCL1 leukemia cells were cultured for 48 hours in the presence of the indicated concentrations of R406. Protein extracts were analyzed for changes in the expression of Mcl-1 and Bim by immunoblotting. Actin served as a loading control.
Sensitivity of TCL1 leukemias to R406-induced apoptosis in vitro. (A) TCL1 leukemia cells were cultured for 48 hours with increasing concentrations of R406. The rate of apoptosis was determined by Annexin V/PI staining. Samples NB1, NB2, and NB3 are normal B cells that were purified by negative selection from the spleens of wild-type mice; samples NS1 and NS2 are mononuclear cells obtained from the spleens of the same animals. (B) Time-course analysis of in vitro sensitivity to R406. The percentage of viable cells relative to control (no R406) was determined in 2 TCL1 leukemias (TCL1-540 and TCL1-551) and 2 normal B-cell samples (NB4 and NB5) after 24, 48, and 72 hours incubation with the indicated concentrations of R406. (C) Human CLL cells and TCL1 leukemia cells were cultured for 48 hours in the presence of the indicated concentrations of R406. Protein extracts were analyzed for changes in the expression of Mcl-1 and Bim by immunoblotting. Actin served as a loading control.
Induction of apoptosis by R406 and other Syk inhibitors in primary human CLL cells has been associated with down-regulation of the antiapoptotic protein Mcl-1.13,20 During the course of this study we observed that R406 also up-regulates the proapoptotic protein Bim in human CLL cells (Figure 2C and data not shown). Induction of Bim was also observed in some of the TCL1 leukemias, but this was not accompanied by a decrease in Mcl-1 expression. Rather, a slight increase in Mcl-1 levels was occasionally observed at higher R406 concentrations, suggesting that expression of this antiapoptotic protein may be subject to different regulatory and, possibly, compensatory mechanisms in TCL1 leukemia cells.
R788 inhibits the growth of adoptively transferred TCL1 leukemias in vivo
Although R406 was not markedly cytotoxic to the leukemic cells in vitro, the possibility remained that this compound could be more active in vivo, where it could potentially inhibit antigen-induced BCR signals. To test this possibility, we investigated the activity of the R406 prodrug R788 against adoptively transferred TCL1 leukemias. The TCL1 leukemias are particularly appropriate for this purpose, since they can be propagated with 100% efficiency in syngeneic mouse recipients,25,30 despite the fact that they are not immortalized and do not grow in vitro. In addition, in a previous study we showed that the BCR of TCL1-002 cells binds to PtC,25 suggesting that at least, in the case of this leukemia, the malignant B cells should be continuously exposed to antigen after adoptive transfer.
To assess the activity of R788 against TCL1-002 leukemia, 1.5 × 107 cells were adoptively transferred in 18 syngeneic recipients by intraperitoneal injection. Three days later treatment was initiated with R788 in 8 mice, whereas the remaining 10 animals were given vehicle control. R788, which is rapidly converted to R406 after intraperitoneal administration, was given during 18 consecutive days at a daily dose of 80 mg/kg (this dose was established as the maximum tolerated dose in previous mouse toxicity studies31 ). Because the plasma half-life of R406 in mice is less than 2 hours (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article),17 we decided to administer R788 in 3 divided doses at 3-hour intervals, so as to provide several hours of continuous Syk inhibition during each day of treatment. We considered that this dosing schedule will more accurately reflect the situation in humans, where Syk inhibition is sustained because of the substantially longer plasma half-life of R406 (15 hours).32
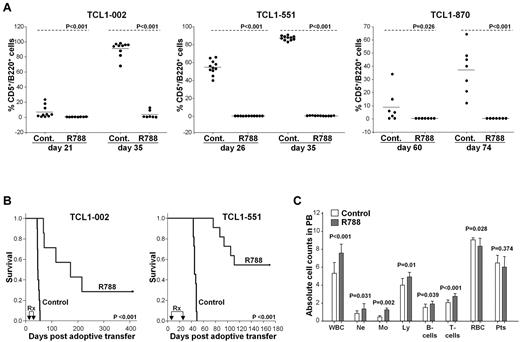
Complete blood counts and flow cytometric analysis were performed on peripheral blood samples on the last day of treatment. At this time point leukemic CD5+/B220+ cells were detectable in the peripheral blood of all animals from the control group, but in none of the animals from the R788 group (Figure 3A left panel and supplemental Figure 2). Two weeks later all mice from the control group had developed overt leukemia (median WBC counts 131 × 106/mL, range 12-300 × 106/mL), whereas WBC counts in the R788-treated group were normal (median 6 × 106/mL, range 3-8 × 106/mL; P < .001), and only a small percentage of leukemic cells was detected in 2 animals. Several weeks later leukemia developed in 3 more mice from the R788 group, whereas 2 mice remained disease free after 400 days of follow-up (one mouse from this group had died on the last day of treatment as a complication of the intraperitoneal injection).
Treatment of adoptively transferred TCL1 leukemias with R788. (A) Analysis of mice with adoptively transferred TCL1-002, TCL1-551, or TCL1-870 leukemia after treatment with R788 or vehicle control. B6/C3H F1 female mice (6-8 weeks old) received 1.5 × 107 leukemia cells by intraperitoneal injection. Three days later treatment was initiated with R788 (80 mg/kg/d) or vehicle control (Cont.). Mice with TCL1-002 leukemia were treated for 18 days, whereas mice with TCL1-551 and TCL1-870 leukemia were treated for 21 days. Peripheral blood samples were collected on the indicated days, and the percentage of malignant CD5+/B220+ cells was determined by flow cytometry. (B) Kaplan-Meier survival curves of mice with adoptively transferred TCL1-002 and TCL1-551 leukemia treated with R788 or vehicle control. The period of treatment (Rx) is indicated by arrows. (C) Complete blood counts in mice with adoptively transferred TCL1-002 leukemia on the last day of treatment. Analysis was performed on a HEMAVET 950 counter. Values represent mean absolute cell counts ± SD × 106/mL for WBCs, Ne, Mo, Ly, and B and T cells, × 109/mL for RBCs, and × 108/mL for Pts (control group n = 10, R788 group n = 8). The absolute number of normal B and T cells was calculated using the percentage of CD5-/B220+ and CD5+/B220− cells from the flow cytometric analysis.
Treatment of adoptively transferred TCL1 leukemias with R788. (A) Analysis of mice with adoptively transferred TCL1-002, TCL1-551, or TCL1-870 leukemia after treatment with R788 or vehicle control. B6/C3H F1 female mice (6-8 weeks old) received 1.5 × 107 leukemia cells by intraperitoneal injection. Three days later treatment was initiated with R788 (80 mg/kg/d) or vehicle control (Cont.). Mice with TCL1-002 leukemia were treated for 18 days, whereas mice with TCL1-551 and TCL1-870 leukemia were treated for 21 days. Peripheral blood samples were collected on the indicated days, and the percentage of malignant CD5+/B220+ cells was determined by flow cytometry. (B) Kaplan-Meier survival curves of mice with adoptively transferred TCL1-002 and TCL1-551 leukemia treated with R788 or vehicle control. The period of treatment (Rx) is indicated by arrows. (C) Complete blood counts in mice with adoptively transferred TCL1-002 leukemia on the last day of treatment. Analysis was performed on a HEMAVET 950 counter. Values represent mean absolute cell counts ± SD × 106/mL for WBCs, Ne, Mo, Ly, and B and T cells, × 109/mL for RBCs, and × 108/mL for Pts (control group n = 10, R788 group n = 8). The absolute number of normal B and T cells was calculated using the percentage of CD5-/B220+ and CD5+/B220− cells from the flow cytometric analysis.
Similar results were obtained with mice challenged with the leukemia lines TCL1-551 and TCL1-870, which expressed low basal levels of phosphorylated Syk. R788 treatment administered from days 4 to 25 after adoptive transfer significantly prevented the outgrowth of these leukemias (Figure 3A middle and right panels). Moreover, 6 of the 11 treated mice with adoptively transferred TCL1-551 cells did not develop leukemia after a considerable follow-up period (almost 6 months), suggesting that R788 treatment can completely eradicate the malignant clone in a substantial proportion of cases.
Treatment with R788 significantly prolonged the survival of the studied animals. Median survival of control group animals with TCL1-002 leukemia was 46 days, whereas median survival of R788-treated animals was 172 days (Figure 3B). Similarly, control group animals with TCL1-551 leukemia had a median survival of 45 days, whereas median survival was not reached at 170 days in the R788 group. The animals with TCL1-870 leukemia were studied more recently and therefore survival data were not available.
Interestingly, although R406 and R788 have been reported to induce a mild transient lymphocytopenia in rats,31 we did not observe a similar reduction in the number of normal B or T cells in the animals treated in our study. Rather, a modest but statistically significant rise in the number of neutrophils, monocytes, T and normal B cells was observed on the last day of R788 treatment (Figure 3C). A likely explanation for this rise was that the different leukocyte subsets had migrated from the tissue compartments to the blood, presumably as a consequence of inhibition of integrin signaling, which is known to be regulated by Syk.33
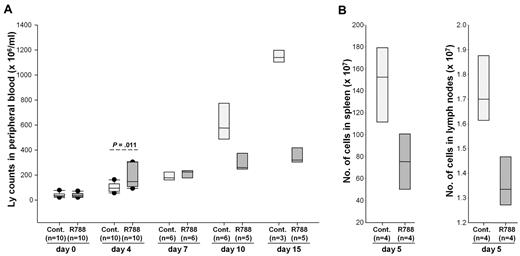
To determine whether R788 would also affect the migration of the malignant lymphocytes, we performed a new experiment in which we monitored changes in circulating leukemia cells during the first 15 days of R788 treatment. In contrast to the previous experiment, this experiment was performed with animals that had detectable leukemia cells in the peripheral blood when treatment was initiated. Interestingly, after 4 days of treatment animals receiving R788 showed a greater rise in the number of circulating malignant lymphocytes than controls (Figure 4A). This rise was associated with a decrease in the number of lymphocytes in spleen and lymph nodes (Figure 4B), suggesting that it was caused by relocation of the malignant cells from the tissues into the blood. Consistent with this explanation, subsequent analysis at days 7, 10, and 15 showed a dramatic rise in the number of circulating leukemic cells in the control group, whereas the number of circulating leukemic cells in the R788 group remained relatively stable. Collectively, these experiments suggest that R788 induces an early and transient mobilization of both normal and malignant B cells, which is subsequently followed by selective inhibition of the growth of the malignant B-cell population.
Effects of R788 on leukemia cell distribution in blood, lymph nodes, and spleen. (A) B6/C3H mice with overt TCL1-002 leukemia were treated for 15 days with R788 or vehicle control. The number of circulating leukemia cells was determined on days 0, 4, 7, 10, and 15. On day 4, animals treated with R788 showed a greater increase in circulating leukemia cells than controls. Subsequently, the number of circulating leukemia cells increased dramatically in animals from the control group, whereas it remained stable in animals from the R788 group. The number of live animals available for analysis at each time point is indicated in the parentheses. (B) Four animals from each group were killed on day 5 to collect spleens and lymph nodes (inguinal and axillary) for analysis of tumor burden. Single-cell suspensions were prepared from the samples and counted on a Hemavet HV950FS hematology analyzer.
Effects of R788 on leukemia cell distribution in blood, lymph nodes, and spleen. (A) B6/C3H mice with overt TCL1-002 leukemia were treated for 15 days with R788 or vehicle control. The number of circulating leukemia cells was determined on days 0, 4, 7, 10, and 15. On day 4, animals treated with R788 showed a greater increase in circulating leukemia cells than controls. Subsequently, the number of circulating leukemia cells increased dramatically in animals from the control group, whereas it remained stable in animals from the R788 group. The number of live animals available for analysis at each time point is indicated in the parentheses. (B) Four animals from each group were killed on day 5 to collect spleens and lymph nodes (inguinal and axillary) for analysis of tumor burden. Single-cell suspensions were prepared from the samples and counted on a Hemavet HV950FS hematology analyzer.
R788 blocks BCR signaling and inhibits leukemic cell survival and proliferation in vivo
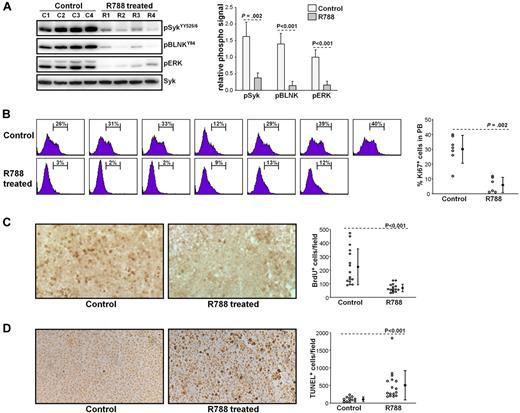
To further explore the mechanisms of R788 action, we performed another set of experiments in which we evaluated the effects of R788 on BCR signaling, leukemic cell proliferation, and survival. These experiments were performed with animals that were allowed to develop overt TCL1-002 leukemias before starting treatment (usually 28 days after adoptive transfer). Animals were sacrificed after 7 days of R788 treatment, and peripheral blood and spleen samples were collected for subsequent analysis. As shown in Figure 5A, R788 effectively inhibited BCR signaling in vivo, as evidenced by a significant reduction in the amount of phosphorylated SykYY525/526, BLNK, and ERK in leukemic cells from R788-treated animals. Inhibition of BCR signaling was associated with a significant reduction in the percentage of proliferating leukemic cells, as evaluated by Ki67 staining and BrdU incorporation (Figure 5B-C). In addition, analysis of spleen sections with the TUNEL assay showed a significant increase in the number of apoptotic cells in R788-treated animals (Figure 5D). Collectively, these experiments suggest that BCR signals are required for both the proliferation and survival of the malignant B cells in vivo.
Effects of R788 treatment on BCR signaling, leukemic cell proliferation, and survival. (A) B6/C3H F1 mice with overt TCL1-002 leukemia were treated for 7 days with R788 or vehicle control. Peripheral blood samples were collected 30 minutes after the last treatment, and immunoblotting analysis was performed on cellular extracts using the indicated antibodies. Changes in the amount of phosphorylated Syk, BLNK, and ERK are summarized in the graph. The phospho-specific signal was calculated as fold difference in signal intensity relative to the signal in sample C1, which was arbitrarily set to 1.0. Values were normalized against total Syk, which was used as a loading control. (B) Ki67 staining was performed on peripheral blood samples from animals with overt TCL1-002 leukemia treated with vehicle control (n = 7) or R788 (n = 6), as described above. The percentage of Ki67-positive cells is indicated in each histogram plot. Pooled data and statistical evaluation are presented in the graph. Open circles represent the percentage of Ki67-positive cells in each animal; mean values and SD are indicated with filled circles and error bars, respectively. Statistical analysis was performed with the Mann-Whitney rank sum test. (C) In vivo BrdU labeling was done in animals with adoptively transferred TCL1-002 leukemia that were treated for 7 days with vehicle control or R788. BrdU was injected twice on day 7 together with R788 or vehicle control, and spleen samples were collected 6 hours later. Representative examples of frozen spleen sections stained with anti-BrdU antibody are shown. BrdU-positive cells are dark brown. The graph summarizes the data from the analysis of 4 mice treated with R788 and 4 mice treated with vehicle control. Four independent fields per spleen were counted using a Widefield Leica DMR microscope and NIH ImageJ software. Open circles represent the number of BrdU-positive cells/field; mean values and SD are indicated with filled circles and error bars. (D) Representative examples of paraffin-embedded spleen sections stained with TUNEL-peroxidase from mice treated for 7 days with R788 or vehicle control. The graph summarizes the data from the analysis of 4 mice treated with R788 and 3 mice treated with vehicle control. Open circles represent the number of TUNEL-positive cells/field; mean values and SD are indicated with filled circles and error bars.
Effects of R788 treatment on BCR signaling, leukemic cell proliferation, and survival. (A) B6/C3H F1 mice with overt TCL1-002 leukemia were treated for 7 days with R788 or vehicle control. Peripheral blood samples were collected 30 minutes after the last treatment, and immunoblotting analysis was performed on cellular extracts using the indicated antibodies. Changes in the amount of phosphorylated Syk, BLNK, and ERK are summarized in the graph. The phospho-specific signal was calculated as fold difference in signal intensity relative to the signal in sample C1, which was arbitrarily set to 1.0. Values were normalized against total Syk, which was used as a loading control. (B) Ki67 staining was performed on peripheral blood samples from animals with overt TCL1-002 leukemia treated with vehicle control (n = 7) or R788 (n = 6), as described above. The percentage of Ki67-positive cells is indicated in each histogram plot. Pooled data and statistical evaluation are presented in the graph. Open circles represent the percentage of Ki67-positive cells in each animal; mean values and SD are indicated with filled circles and error bars, respectively. Statistical analysis was performed with the Mann-Whitney rank sum test. (C) In vivo BrdU labeling was done in animals with adoptively transferred TCL1-002 leukemia that were treated for 7 days with vehicle control or R788. BrdU was injected twice on day 7 together with R788 or vehicle control, and spleen samples were collected 6 hours later. Representative examples of frozen spleen sections stained with anti-BrdU antibody are shown. BrdU-positive cells are dark brown. The graph summarizes the data from the analysis of 4 mice treated with R788 and 4 mice treated with vehicle control. Four independent fields per spleen were counted using a Widefield Leica DMR microscope and NIH ImageJ software. Open circles represent the number of BrdU-positive cells/field; mean values and SD are indicated with filled circles and error bars. (D) Representative examples of paraffin-embedded spleen sections stained with TUNEL-peroxidase from mice treated for 7 days with R788 or vehicle control. The graph summarizes the data from the analysis of 4 mice treated with R788 and 3 mice treated with vehicle control. Open circles represent the number of TUNEL-positive cells/field; mean values and SD are indicated with filled circles and error bars.
R788 inhibits the growth of spontaneously developing TCL1 leukemias
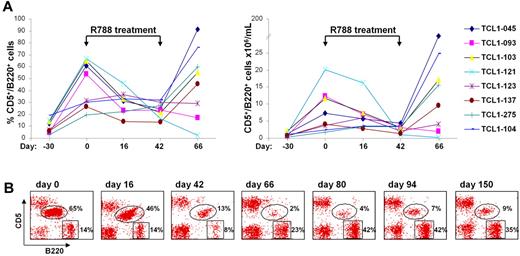
We next investigated whether R788 would also be effective against spontaneously developing TCL1 leukemias. For this experiment we selected 8 Eμ-TCL1 transgenic mice that before treatment showed a steady rise in both the percentage and absolute number of CD5+/B220+ cells in the peripheral blood (Figure 6A). The CD5+/B220+ expansion was monoclonal in all animals, as evidenced by gene scan analysis of IgVH gene rearrangements (data not shown). After 16 days of R788 treatment a significant decrease in the percentage and number of CD5+/B220+ cells was observed in 5 animals, with a further reduction by the last day of treatment (day 42). The disease remained stable in one animal (TCL1-123), whereas it continued to progress under therapy in 2 other mice (TCL1-275 and TCL1-104). One month after therapy was discontinued the number of leukemic cells rose to pretreatment or higher levels in 6 animals, whereas they remained low or continued to fall in 2 mice (TCL1-093 and TCL1-121, respectively). In TCL1-121 there was an almost complete disappearance of the leukemic CD5+/B220+ clone by day 66, with a concomitant rise in the percentage of normal B cells (Figure 6B). However, on follow-up analysis the percentage of CD5+/B220+ cells started to rise again, suggesting that the malignant clone was not eradicated in any animal from this cohort.
Treatment of spontaneous TCL1 leukemias with R788. (A) 8 Eμ-TCL1 transgenic mice that had developed spontaneous leukemia were treated for 42 days with R788. Shown are the percentage and absolute number of malignant CD5+/B220+ cells before, during and after therapy. (B) Flow cytometric analysis of peripheral blood samples from mouse TCL1-121 collected before (day 0), during (days 16 and 42), and after therapy (days 66, 80, 94, and 150).
Treatment of spontaneous TCL1 leukemias with R788. (A) 8 Eμ-TCL1 transgenic mice that had developed spontaneous leukemia were treated for 42 days with R788. Shown are the percentage and absolute number of malignant CD5+/B220+ cells before, during and after therapy. (B) Flow cytometric analysis of peripheral blood samples from mouse TCL1-121 collected before (day 0), during (days 16 and 42), and after therapy (days 66, 80, 94, and 150).
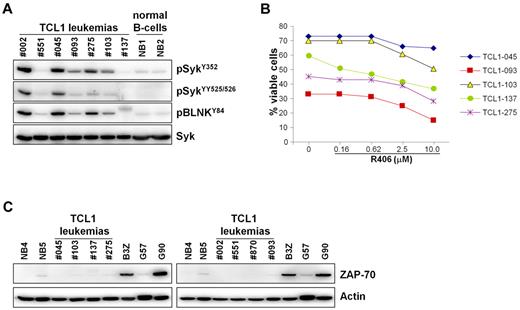
In an attempt to identify features that could predict the response to R788 therapy, we determined the IgVH nucleotide sequence of all TCL1 leukemias that were treated with R788 in vivo (Table 1). However, no association was found between response to R788 treatment and IgVH mutation status or IgVH gene usage. All TCL1 leukemias were found to express unmutated IgVH genes and there was no bias in IgVH gene use that could suggest a difference in antigen specificity between the responding and nonresponding cases. Thus, 2 of the 4 TCL1 leukemias that expressed BCRs with VH and CDR3 features typical of anti-PtC antibodies27,28 were sensitive to R788 treatment (TCL1-002 and TCL1-045), whereas the other 2 were resistant (TCL1-104 and TCL1-275). In addition, TCL1-121 and TCL1-123 expressed BCRs that were encoded by the same IgVH gene (V1-52) associated with very similar HCDR3 sequences, but differed in their response to R788. The response to R788 treatment also did not correlate with the amount of phospho-Syk or the in vitro sensitivity to R406, which was equal between the responding and nonresponding cases (Figure 7A-B; Table 1). In addition, all TCL1 leukemias were ZAP-70-negative and CD38-positive, suggesting that the response to R788 treatment is not related to the expression of these 2 important prognostic factors (Figure 7C; Table 1; supplemental Figure 3). Collectively, these data suggest that other factors underlie the resistance of some of these leukemias to R788 treatment.
Features of TCL1 leukemias treated with R788 in vivo
| Leukemia . | Model . | Sex . | VH gene . | D . | JH . | CDR3 . | Ag specificity . | phospho-SykY352 . | ZAP-70 . | CD38 . | Response to R788 therapy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (% hom) . | |||||||||||
| TCL1-002 | Adoptive transfer | Female recipients | V12S1 (100%) | Q52 | J1 | CAG-DRTGYWYFDV-W | Confirmed anti-PtC | Strong | neg. | pos. | Yes |
| TCL1-551 | Adoptive transfer | Female recipients | V7-3 (100%) | D1-1 | J4 | CAR-YYYGSSYAMDY-W | Unknown | Weak | neg. | pos. | Yes |
| TCL1-870 | Adoptive transfer | Female recipients | V1-55 (100%) | D1-1 | J1 | CAR-RYYGSSYWYFDV-W | Unknown | Weak | neg. | pos. | Yes |
| TCL1-137 | Spontaneous | Female | V1-55 (100%) | D1-1 | J1 | CAR-IYYGSSYWYFDV-W | Unknown | Weak | neg. | pos. | Yes |
| TCL1-045 | Spontaneous | Female | V11-2 (100%) | D2-3 | J1 | CMR-YDGYYWYFDV-W | VH sequence homology with anti-PtC Abs | Strong | neg. | pos. | Yes |
| TCL1-093 | Spontaneous | Male | V6-6 (100%) | D3-3 | J2 | CTT-SLRL-W | Unknown | Moderate | neg. | pos. | Yes |
| TCL1-103 | Spontaneous | Male | V1-80 (100%) | D2-11 | J2 | CAR-SWYYFDY-W | Unknown | Moderate | neg. | pos. | Yes |
| TCL1-121 | Spontaneous | Male | V1-52 (100%) | D2-1 | J1 | CAI-YYGNYWYFDV-W | Unknown | n.d. | n.d. | n.d. | Yes |
| TCL1-123 | Spontaneous | Female | V1-52 (99.6%) | D1-1 | J1 | CAS-YGSSYWYFDV-W | Unknown | n.d. | n.d. | n.d. | No |
| TCL1-104 | Spontaneous | Female | V11-2 (100%) | D2-1 | J1 | CMR-YGNYWYFDV-W | VH sequence homology with anti-PtC Abs | n.d. | n.d. | n.d. | No |
| TCL1-275 | Spontaneous | Male | V11-2 (100%) | D2-1 | J1 | CMR-YGNYWYFDV-W | VH sequence homology with anti-PtC Abs | Moderate | neg. | pos. | No |
| Leukemia . | Model . | Sex . | VH gene . | D . | JH . | CDR3 . | Ag specificity . | phospho-SykY352 . | ZAP-70 . | CD38 . | Response to R788 therapy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (% hom) . | |||||||||||
| TCL1-002 | Adoptive transfer | Female recipients | V12S1 (100%) | Q52 | J1 | CAG-DRTGYWYFDV-W | Confirmed anti-PtC | Strong | neg. | pos. | Yes |
| TCL1-551 | Adoptive transfer | Female recipients | V7-3 (100%) | D1-1 | J4 | CAR-YYYGSSYAMDY-W | Unknown | Weak | neg. | pos. | Yes |
| TCL1-870 | Adoptive transfer | Female recipients | V1-55 (100%) | D1-1 | J1 | CAR-RYYGSSYWYFDV-W | Unknown | Weak | neg. | pos. | Yes |
| TCL1-137 | Spontaneous | Female | V1-55 (100%) | D1-1 | J1 | CAR-IYYGSSYWYFDV-W | Unknown | Weak | neg. | pos. | Yes |
| TCL1-045 | Spontaneous | Female | V11-2 (100%) | D2-3 | J1 | CMR-YDGYYWYFDV-W | VH sequence homology with anti-PtC Abs | Strong | neg. | pos. | Yes |
| TCL1-093 | Spontaneous | Male | V6-6 (100%) | D3-3 | J2 | CTT-SLRL-W | Unknown | Moderate | neg. | pos. | Yes |
| TCL1-103 | Spontaneous | Male | V1-80 (100%) | D2-11 | J2 | CAR-SWYYFDY-W | Unknown | Moderate | neg. | pos. | Yes |
| TCL1-121 | Spontaneous | Male | V1-52 (100%) | D2-1 | J1 | CAI-YYGNYWYFDV-W | Unknown | n.d. | n.d. | n.d. | Yes |
| TCL1-123 | Spontaneous | Female | V1-52 (99.6%) | D1-1 | J1 | CAS-YGSSYWYFDV-W | Unknown | n.d. | n.d. | n.d. | No |
| TCL1-104 | Spontaneous | Female | V11-2 (100%) | D2-1 | J1 | CMR-YGNYWYFDV-W | VH sequence homology with anti-PtC Abs | n.d. | n.d. | n.d. | No |
| TCL1-275 | Spontaneous | Male | V11-2 (100%) | D2-1 | J1 | CMR-YGNYWYFDV-W | VH sequence homology with anti-PtC Abs | Moderate | neg. | pos. | No |
hom indicates homology; n.d., not determined; neg., negative; and pos., positive.
Syk activity, sensitivity to R406-induced apoptosis, and ZAP-70 expression in TCL1 leukemias treated with R788. (A) Leukemic cells were isolated from the spleens of Eμ-TCL1 transgenic mice 1-2 months after treatment with R788 had been discontinued. TCL1 leukemias that were used in the adoptive-transfer experiments (TCL1-002 and TCL1-551) and normal B-cell samples (NB1 and NB2) were loaded as controls. Syk activity was evaluated by immunoblotting analysis with phospho-Syk and phospho-BLNK antibodies. (B) Percentage of viable leukemic cells (annexin V/PI negative) after 48 hours in culture with R406. (C) Immunoblotting analysis of ZAP-70 expression in TCL1 leukemias. Leukemia cells were purified by negative selection, the percentage of remaining T cells after purification was less than 3%. Negative controls included 2 normal B-cell samples purified by positive selection from spleens of wild-type mice (NB4 and NB5) and 1 ZAP-70–negative CLL sample (G57). Positive controls were the mouse T-cell line B3Z and 1 ZAP-70–positive CLL sample (G90). The CLL samples were purified by negative selection, as described elsewhere.15 Actin served as a loading control.
Syk activity, sensitivity to R406-induced apoptosis, and ZAP-70 expression in TCL1 leukemias treated with R788. (A) Leukemic cells were isolated from the spleens of Eμ-TCL1 transgenic mice 1-2 months after treatment with R788 had been discontinued. TCL1 leukemias that were used in the adoptive-transfer experiments (TCL1-002 and TCL1-551) and normal B-cell samples (NB1 and NB2) were loaded as controls. Syk activity was evaluated by immunoblotting analysis with phospho-Syk and phospho-BLNK antibodies. (B) Percentage of viable leukemic cells (annexin V/PI negative) after 48 hours in culture with R406. (C) Immunoblotting analysis of ZAP-70 expression in TCL1 leukemias. Leukemia cells were purified by negative selection, the percentage of remaining T cells after purification was less than 3%. Negative controls included 2 normal B-cell samples purified by positive selection from spleens of wild-type mice (NB4 and NB5) and 1 ZAP-70–negative CLL sample (G57). Positive controls were the mouse T-cell line B3Z and 1 ZAP-70–positive CLL sample (G90). The CLL samples were purified by negative selection, as described elsewhere.15 Actin served as a loading control.
Discussion
Chronic or recurrent antigen stimulation is considered to play a key role in the development and progression of several B-cell malignancies, including CLL, Helicobacter pylori–associated gastric mucosa-associated lymphatic tissue lymphomas, and certain indolent lymphomas associated with chronic hepatitis C virus infection.1-3,34-36 In the case of H. pylori and hepatitis C virus–associated lymphomas the pathogenic role of antigen is further corroborated by clinical studies, which have shown that elimination of the infectious agent can lead to tumor regression in patients with early stages of the disease.37-39 Although attractive, this approach cannot be applied to CLL because no microbial agent has yet been associated with this disease. Rather, emerging evidence suggests that the malignant cells frequently bind to autoantigens exposed on the surface of apoptotic cells, thus suggesting that antigen eradication will not be a feasible therapeutic strategy in CLL.40-42
An alternative strategy to disrupt antigen-receptor signaling in CLL would be to block BCR signal transduction using small-molecule inhibitors. This approach was tested in the current study using the selective Syk inhibitor R788 and the Eμ-TCL1 transgenic mouse model of CLL. The leukemias that develop in Eμ-TCL1 transgenic mice are currently the most widely used preclinical model to study the activity of novel therapeutic agents for CLL.30,43-45 There is mounting evidence that these leukemias are antigen driven, suggesting that they should be especially suitable for testing agents that target the BCR signaling pathway. As is the case with human CLL, these leukemias frequently express stereotyped BCRs that bind to neoantigens exposed on the surface of senescent or apoptotic cells,25 which was also observed in our present study. In addition, recent studies have shown that disruption of genes involved in BCR signal transduction prevents or significantly delays the development of these leukemias, further suggesting a role for antigen stimulation during the expansion of the malignant clones.46,47
In the current study we show that R788 is highly active in 2 Eμ-TCL1 leukemia models, (that is, adoptively transferred TCL1 leukemias and leukemias that spontaneously develop in Eμ-TCL1 transgenic mice). Treatment with R788 inhibited both the proliferation and survival of the malignant B cells, leading to eradication of the malignant clones in a considerable proportion of animals with adoptively transferred leukemia. It is noteworthy that these effects were observed after relatively short treatments with R788, lasting from 18 to a maximum of 45 days.
The primary mechanism of R788 action appeared to be inhibition of antigen-dependent BCR signaling, rather than inhibition of constitutive Syk activity, for several reasons. First, R788 was equally effective against leukemias with high or low basal levels of phosphorylated Syk, including leukemias that expressed the same amount of active Syk as normal B cells. Second, R788 selectively inhibited the growth of the malignant clones and had almost no effect on the normal B-cell population, despite the equal or even greater sensitivity of the latter to the cytotoxic effect of this compound in vitro. Third, the cytotoxic effect of R406 in vitro was relatively modest and occurred at high R406 concentrations to which the cells were exposed continuously for 48 hours. These concentrations were reached only intermittently in vivo, which could explain the absence of a significant cytopenic effect on the normal B-cell population.
The arguments presented above do not entirely exclude the possibility that the therapeutic effect of R788 was, to some extent, also due to the inhibition of antigen-independent BCR signals. In this respect, R788 was recently shown to reduce the tumor burden and prolong the survival of mice transplanted with non-Hodgkin lymphomas (NHLs) that develop in the absence of antigen stimulation (Eμ-MYC/IgHEL transgenic mice), suggesting that inhibition of the tonic BCR signal could be therapeutically effective in certain B-cell malignancies.17 It is worth noting, however, that R788 was ineffective in preliminary experiments with the TCL1-002 leukemia when the drug was administered once a day, as in the case of the Eμ-MYC/IgHEL study, or twice a day at 8- to 10-hour intervals (data not shown). Rather, in the case of the TCL1 leukemias, repeated administrations of the drug providing several hours of continuous Syk inhibition were required for growth inhibition, suggesting that the BCR signals targeted by R788 in these 2 models were qualitatively and/or quantitatively different.
R788 was recently tested in phase II clinical trials of rheumatoid arthritis, immune thrombocytopenic purpura, and recurrent B-cell NHL, where it showed considerable clinical activity in the absence of significant toxicities or side effects.48-50 Interestingly, the NHL trial included a small series of patients with small lymphocytic leukemia/CLL, which showed the highest response rate (55%).50 This study also showed that normal B cells are not affected by R788 in vivo, in parallel to the situation observed in our mouse model.
The encouraging data from the NHL clinical trial, together with the results of our current study, suggest that CLL should be a particularly appropriate setting for future clinical trials with R788. There is substantial rationale to investigate R788 in combination with chemotherapy, considering that BCR engagement can increase the resistance of the leukemic cells to fludarabine-induced apoptosis11 and can enhance their homing and retention to the protective tissue microenvironments.18 Another approach worth investigating would be to use R788 as consolidation therapy, especially considering that this compound appeared particularly effective in treating TCL1 leukemias with low tumor burden.
Leukemias resistant to R788 were observed both in the clinical trial by Friedberg et al50 and in our animal model. The nonresponding TCL1 leukemias did not appear to differ in antigen specificity from the responding cases, since, in both instances, leukemias that expressed BCRs with features typical of anti-PtC antibodies were identified. In addition, all TCL1 leukemias expressed unmutated IgVH genes and were ZAP-70–negative and CD38-positive, suggesting that the response to R788 is not related to the expression of these prognostic factors. Thus, other factors will have to be considered to account for the variability in the response, such as mutations that render the malignant clones independent of BCR signals or differences in the degree of Syk inhibition. The latter possibility requires consideration particularly in the animal model, given the short plasma half-life of R406 in mice and the apparent requirement for sustained Syk inhibition. In the NHL trial no correlation between R406 pharmacokinetics and clinical outcome was detected,50 but pharmacokinetic analysis in a few patients from the ITP trial indicated a correlation between the degree of Syk inhibition and platelet response.49 Thus, further pharmacokinetic and pharmacodynamic studies appear warranted and may provide useful information on the mechanisms that determine the response to R788.
In summary, the data presented in this study demonstrate that disruption of BCR signaling can selectively inhibit the proliferation and survival of the malignant B cells in the Eμ-TCL1 transgenic mouse model of CLL. Considering the similarities between the mouse model and the human disease, these data provide a further rationale for clinical trials with R788 in CLL and establish the BCR-signaling pathway as a potential therapeutic target in this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Carlo Croce for kindly providing the Eμ-TCL1 transgenic mice and Drs Stefania Gobessi, Daniel Bilbao-Cortes, Yasumichi Hitoshi, and Polly Pine for their helpful comments and discussions.
This work was supported by grants from the Italian Association for Cancer Research (Milan, Italy, no. 5917) and The Leukemia & Lymphoma Society (White Plains, NY, no. R6170-10).
Authorship
Contribution: M.S. designed and performed most of the experiments, analyzed and interpreted data, and wrote the manuscript; P.G.L. and S.B. designed and performed experiments; E.P. performed the histologic analysis; G.L. and L.L. provided intellectual input into the design of the study and reviewed the manuscript; and D.G.E. designed the research and experiments, reviewed all of the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitar G. Efremov, ICGEB Outstation-Monterotondo, CNR Campus “Adriano Buzzati-Traverso,” Via E. Ramarini 32, I-00016 Monterotondo Scalo (Rome), Italy; e-mail: efremov@icgeb.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal