The anti-inflammatory effects of IVIg have been adoptively transferred using IVIg-primed cells in autoimmunity and inflammatory states (reviewed in Crow et al1 and Lazarus2 ). Although dendritic cells (DCs) have entered the spotlight in this process, the downstream target of these cells has been unknown. In this issue of Blood, Huang and colleagues observe that IVIg-primed DCs can surprisingly induce downstream regulatory effects in murine ITP at the level of the platelet rather than the phagocyte.3
Intravenous immunoglobulin (IVIg) has beneficial effects in a wide array of inflammatory states and autoimmune diseases. Although much remains to be unveiled about all of the details surrounding the mechanistic effects of IVIg, it has been proposed that activating Fcγ receptors on the surface of DCs are a primary target of IVIg.4 A specific role for FcγRIII-dependent signaling has also been implicated in IVIG action in murine immune thrombocytopenia (ITP).5 What has been missing is an understanding of the downstream pathway whereby these IVIg-primed DCs mediate their inhibitory effects. Although the downstream mechanistic pathway induced by DCs is likely complex, the general assumption was that platelets were only the victims in ITP and that the target of this immune regulation would more logically affect the macrophage. In sharp contrast to this“default” thinking, Huang and coworkers now demonstrate that IVIG-primed DCs mediate an anti-inflammatory activity at the level of the platelet itself rather than an effect on the phagocytic cell in a murine model of ITP (see figure).
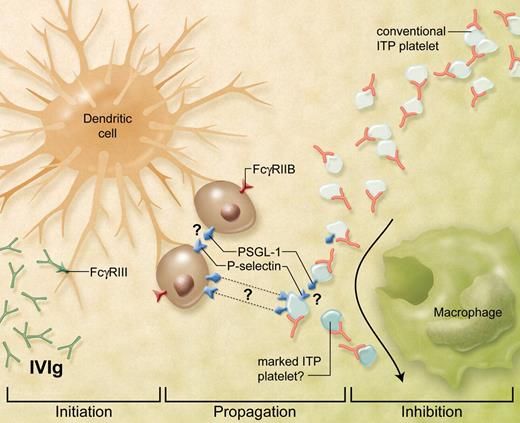
IVIg-directed DC-platelet nuptials. IVIg effects in autoimmunity and inflammation can be mediated by DCs. IVIg first primes the initiator DCs4 in an FcγRIII-dependent manner.3,5 These cells then propagate a still-poorly described intermediary pathway which may involve the function of several interacting cells. The propagation and/or inhibition stages appear to be dependent on the presence of FcγRIIB and P-selectin. The specific cell that expresses these molecules in terms of IVIg action remains unclear. Platelets have only been thought of as passive victims in ITP, however, Huang et al now show that an important downstream target affected by IVIg-primed DCs could be the platelet rather than the macrophage. A result of this IVIg pathway is the “marking” of the ITP platelet which has reduced ability to engage the phagocytic Mϕ. How the platelet is exactly affected remains unknown but could involve some modification of the platelet or a potential involvement of P-selectin or its ligand PSGL-1. (Professional illustration by Alice Y. Chen.)
IVIg-directed DC-platelet nuptials. IVIg effects in autoimmunity and inflammation can be mediated by DCs. IVIg first primes the initiator DCs4 in an FcγRIII-dependent manner.3,5 These cells then propagate a still-poorly described intermediary pathway which may involve the function of several interacting cells. The propagation and/or inhibition stages appear to be dependent on the presence of FcγRIIB and P-selectin. The specific cell that expresses these molecules in terms of IVIg action remains unclear. Platelets have only been thought of as passive victims in ITP, however, Huang et al now show that an important downstream target affected by IVIg-primed DCs could be the platelet rather than the macrophage. A result of this IVIg pathway is the “marking” of the ITP platelet which has reduced ability to engage the phagocytic Mϕ. How the platelet is exactly affected remains unknown but could involve some modification of the platelet or a potential involvement of P-selectin or its ligand PSGL-1. (Professional illustration by Alice Y. Chen.)
DCs purified from the spleens of naive mice were incubated in vitro with IVIg and adoptively transferred into recipient ITP mice, similar to a previous study.4 After 24 hours, the degree of thrombocytopenia was assessed in the recipient mice, the spleen removed, and a binding interaction assay performed with CD14+ monocyte/macrophages (Mϕ) and opsonized platelets from selected groups of mice. The authors assessed the ability of Mϕ from IVIg-DC–primed mice versus Mϕ from naive mice to engage opsonized platelets from IVIg-DC–primed mice versus those from naive mice. This novel approach allowed the authors to map whether the downstream effects of IVIg-DC priming orchestrate a change in the attributes of the phagocytic Mϕ versus the platelet. Surprisingly, only under conditions where the platelets were isolated from mice treated with IVIg-primed DCs did the number of interactions between the opsonized platelets and Mϕ decrease. This is the first clear indication that IVIg targets (or marks) the platelets rather than phagocytic cells.
Although details on how the platelets are marked or affected by this IVIg pathway remain to be solved, IVIg appeared to have an absolute requirement for P-selectin in the recipient mice.3 P-selectin or P-selectin glyco-protein ligand-1 (PSGL-1) expression by platelets, DCs, or other cells involved in IVIg action (such as endothelial cells6 ) could interact together to either further complicate these IVIg-directed nuptials or help provide clues as to how or why IVIg works in the myriad of diseases where it is beneficial.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal