In this issue of Blood, Bastien and colleagues demonstrate that photopheresis purges donor T cells proliferating against host alloantigens in patients with GVHD after HSCT.1 Photodepletion of these alloreactive lymphocytes concomitantly preserves and expands Tregs, which when reinfused into patients with treatment-refractory chronic GVHD, survive in vivo and correlate with positive clinical responses.
This group previously demonstrated that infusion of photodepleted donor lymphocytes after haploidentical, allogeneic hematopoietic stem cell transplantation (HSCT) reduced posttransplantation infectious complications, but did not induce graft-versus-host disease (GVHD).2 They now raise the bar and successfully explore mechanisms and treatment of patients with established chronic GVHD, using donor lymphocytes depleted of alloreactive CD4+ effector T cells and enriched for regulatory T cells (Tregs).1 Clinical trials of extracorporeal photopheresis (ECP) for refractory chronic GVHD have yielded some encouraging results, yet conclusive immune mechanisms to account for the observed effects have not emerged.3-5
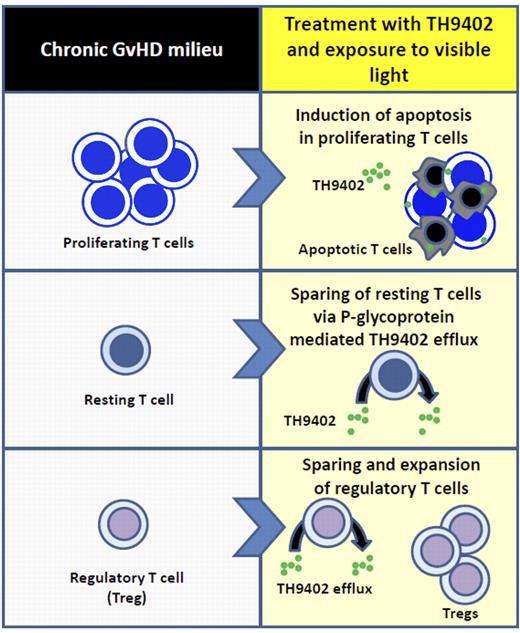
Unlike conventional photodepletion, Bastien and colleagues performed ECP using the rhodamine-derived photosensitizing agent, TH9402. Rhodamine-derived photosensitizers differ from standard psoralen-based compounds, as they are activated by visible light, instead of nonionizing ultraviolet (UV) radiation. TH9402-based photodepletion involves the collection of patient lymphocytes, treatment of the cells ex vivo with TH9402, followed by exposure of the lymphocytes to a visible light source. The photodepleted cells are then reinfused into the patient host with autologous serum. Previously, this group used healthy donor peripheral blood mononuclear cells (PBMCs) and showed that TH9402 induces mitochondrial oxidative damage and cell death in proliferating CD4+ and CD8+ T cells.6 Resting T cells are spared from the effects of photodepletion, as they can eliminate TH9402 through a P-glycoprotein–dependent mechanism. This drug efflux mechanism is regulated by multidrug-resistance-1 (MDR1) gene expression, which is inactive in proliferating T cells.
Bastien et al again “up the ante” by demonstrating in a human study that ECP eliminates proliferating donor T cells not only in healthy donor samples, but also from patients with chronic GVHD (see figure).1 Their current analysis advances our understanding of ECP in chronic GVHD by demonstrating that photodepletion with TH9402 preserves the immunosuppressive capacity of Tregs and allows for their expansion. Photodepletion resulted in a doubling of Foxp3+ cells among the treated PBMCs. Treg proliferation per se did not account for their proportional increase among photodepleted compared with untreatedPBMCs. Rather, exposure to photodepletion-preserved Tregs was necessary and sufficient to induce the conversion of CD4+CD25− Foxp3− T cells to CD4+CD25+ Foxp3+ Tregs. Cell-cell contact involving CTLA4 expressed on Tregs proved to be a critical initiating event leading to IL-10 secretion, which supported this conversion.
As with many studies involving GVHD, this one also focuses on the effector arm of the immune response. An important unanswered question in this study, however, is whether TH9402-sensitized photopheresis also affects the other half of this equation, which is the afferent sensitization of donor T cells at the level of antigen presentation. Dendritic cells (DCs) are a heterogeneous group of highly potent, bone marrow–derived, antigen-presenting cells (APCs) that direct the balance of peripheral tolerance and T-cell sensitization.7 Important remaining unknowns therefore include the extent to which this and other forms of ECP may alter the number and function of circulating DCs, how ECP affects donor DC cross-presentation of host antigens to donor T cells, whether effects on circulating DCs translate to effects in GVHD target organs and tissues, and whether the consequences of ECP vary between different DC subsets and maturation states.
Among other putative mechanisms, for example, photodepletion-preserved DCs may mediate the observed increase in Foxp3+ Tregs through an indoleamine 2,3-dioxygenase (IDO) mechanism, as IDO-expressing conventional DCs can significantly expand Tregs in autologous or HLA-matched human systems.8 One could speculate that a similar mechanism supports the beneficial effects on experimental GVHD in mice when mouse DCs increase IDO after exposure to histone deacetylase inhibitors.9 Harnessing the graft-versus-tumor (GVT) effect of the transplant, by restricting graft alloreactivity to antigens unique to the tumor and not shared with normal host tissues (thereby reducing GVHD), remains one of the overarching goals in the field. Although the current report does not yet provide data regarding relapse in treated patients with chronic GVHD, mouse models suggest that Tregs can help maintain protection against GVHD without compromising GVT.10,11 Investigators still have much to learn about the complete immunosuppressive mechanisms underlying ECP, although altered function of DCs and other APCs likely plays a critical role. For a complication like treatment-refractory GVHD, the field of allogeneic HSCT eagerly awaits more detailed and long-term results of the incipient clinical trial informed by the careful laboratory-based investigations undertaken by Bastien and colleagues.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■