Immune thrombocytopenia (ITP) is an autoantibody-mediated bleeding disorder with both accelerated platelet destruction and impaired platelet production. We and others have described impaired regulatory CD4+CD25hi T cells (Treg) numbers and/or suppressive function in ITP patients. Clinical trials using thrombopoietic agents to stimulate platelet production have shown favorable outcomes in ITP patients, but information on the immunologic responses of treated patients are lacking. We studied the immunologic profile of chronic ITP patients before (n = 10) and during treatment with thrombopoietin receptor (TPO-R) agonists (n = 9). Treg activity, as measured by suppression of proliferation of autologous CD4+ CD25− cells, was improved in patients on treatment (P < .05), and the improvement correlated with reduction in interleukin-2–producing CD4+ cells, consistent with dampening of immune responses. There was a concomitant increase in total circulating transforming growth factor-β1 (TGF-β1) levels (P = .002) in patients on treatment, and the levels of TGF-β1 correlated with the degree of improvement in platelet counts (r = .8, P = .0002). This suggests that platelets in patients on TPO-R treatment may play a role in improving Treg function, either directly or indirectly by enhanced release of TGF-β1 as a result of greater platelet turnover. In conclusion, our findings suggest that thrombopoietic agents in patients with ITP have profound effects to restore immune tolerance.
Introduction
Immune thrombocytopenia (ITP) is a bleeding disorder resulting from low platelet counts with an incidence of 2 and 12 per 100 000 adults and children, respectively, per year and a mortality rate of 1% to 3% per year in severely affected cases.1,2 Autoreactive antibodies to platelet antigens, mainly the platelet glycoprotein IIb/IIIa complex, are considered responsible for accelerated destruction of platelets by the reticuloendothelial system and also reduced platelet production.3 Whereas healthy persons harbor platelet-specific autoreactive T cells that are tolerized in the periphery,4 patients with ITP possess activated platelet-autoreactive T cells with increasing cytokine imbalance toward interleukin-2 (IL-2) and interferon-γ,5,,,–9 especially in patients with chronic ITP with some also reporting higher levels of circulating proinflammatory cytokines tumor necrosis factor-α10 and soluble CD40 ligand (sCD40L).11 These data are consistent with loss of peripheral tolerance and an inflammatory phenotype in chronic ITP patients.
CD4+ regulatory T cells (Tregs) play a critical role in maintenance of peripheral tolerance by both directly and indirectly suppressing the activation and proliferation of many cell types, including T cells, B cells, dendritic cells, natural killer cells, and natural killer T cells in vivo and/or in vitro.12 Because of their ability to control homeostasis and immunopathology,13 the level of Tregs and their function are among the most informative criteria of a patient's immune status. Tregs are characterized by high expression of the CD25 molecule (the IL-2 receptor α-chain) and expression of the transcription factor Foxp3 and make up 5% to 10% of the normal peripheral CD4+ T-cell population.14,15 As with a number of other autoimmune diseases, recent studies in patients with ITP have shown reduced levels of Foxp3 mRNA16 and protein17 in circulating mononuclear cells and abnormal Treg function in spleen biopsies.18 We recently showed that circulating Treg-suppressive activity was reduced in patients with chronic ITP and that the defect was intrinsic to Tregs rather than a result of effector T cells resisting suppression.19 These studies indicate that deficiency in generation and/or defective functions of Tregs may contribute to the loss of immunologic self-tolerance and pathogenesis in patients with ITP. In particular, failure to maintain immune suppression by Tregs may explain the reported platelet autoantigen-specific T-cell proliferative responses and the proinflammatory phenotype in ITP patients. Interestingly, chronic ITP patients treated with rituximab whose platelet counts improve show restored numbers of Tregs as well as restored regulatory activity as determined by in vitro cell proliferation assays.20 Similarly, improvement in Treg frequency and activity has been reported after treatment with high-dose dexamethasone in patients with ITP,17 and in vitro studies indicate a positive effect of intravenous immunoglobulin on Treg function.21 Altogether, the data are consistent with the immunomodulatory nature of such treatment modalities in the setting of chronic ITP.
More recently, a number of thrombopoietic agents have been developed and shown to be highly effective in the treatment of ITP.22 These agents include a thrombopoietic mimetic containing the thrombopoietin receptor (TPO-R)–activating peptides attached to the Fc portion of IgG (Nplate or romiplostim or AMG 531) given as weekly subcutaneous injections, and small molecule TPO-R agonists administered as daily oral tablets (eltrombopag or Promacta; AKR-501; and LGD4665). They increase thrombopoiesis by activating TPO-R, thereby increasing the production of mature megakaryocytes and platelets.23 Romiplostim and eltrombopag have completed phase 1 to 3 clinical trials, and more than 70% of patients had achieved “safe” levels of platelet counts that prevent bleeding (≥ 50 × 109/L) with favorable outcomes with respect to safety and tolerability with both drugs.24,,,–28 AKR-501 has completed a phase 1 trial, demonstrating an increase of more than 50% over the baseline platelet count in 5 of 6 healthy volunteers tested,29 and additional clinical trials in patients with chronic ITP are under way. No information is currently available on the immune responses of patients treated with any of these drugs as it relates to prognosis and response to treatment.
In this study, we analyzed the immunologic profiles of a cohort of patients before treatment and in patients who had been on treatment with thrombopoietic agents. Surprisingly, we found an improvement in the Treg activity of patients on treatment and a decrease in their proinflammatory sCD40L with a concomitant increase in their circulatory transforming growth factor-β1 (TGF-β1), which correlated with an increase in platelet counts. These data suggest that thrombopoietic agents possess immunomodulatory activity as indicated by the improved peripheral Treg function in patients on treatment and an accompanying decrease in inflammatory state. In addition, the data raise the interesting possibility that the increase in platelet counts may play a role in mediating the increase in Treg function in patients on treatment.
Methods
Patient population
All the studies were approved by the institutional review boards of the Weill Medical College of Cornell University and the New York Blood Center. Peripheral blood was obtained from 17 patients (all white) with chronic ITP study (Table 1) and 9 healthy normal healthy volunteers as controls upon informed consent in accordance with the Declaration of Helsinki. In 2 cases (patients 9 and 10), we were able to sample blood from the same patient before they started treatment with thrombopoietic agents (“pretreatment”) and after 4 months on treatment (Table 1). The “pretreatment” group (total n = 10, including patients 9 and 10) consisted of 8 females and 2 males with median age 58.5 years (range, 17-70 years) who had completed their previous treatment (nonthrombopoietic agents) at least 2 weeks before their study visit (Table 1). The “on-treatment” group (total n = 9, including patients 9 and 10) consisted of 8 females and 1 male with median age 54 years (range, 46-80 years) who had been on treatment with thrombopoietic agents (with romiplostim, n = 4; eltrombopag, n = 1; or an investigational thrombopoietic agent AKR-501, n = 4) for at least 4 months at the time of the visit (Table 1).
Demographic and clinical characteristics of chronic ITP patients before and during treatment with thrombopoietic agents
| Patient no. . | Age, y . | Sex . | Splenectomy . | Platelets, ×109/L . | Thrombopoietic agent (time on treatment) at time of blood sampling/collection . | Previous/ongoing non-TPO-R agonist treatment type at time of blood sampling/collection . | |
|---|---|---|---|---|---|---|---|
| Pretreatment | |||||||
| 1 | 67 | Female | Yes | 29 | None | 5 mo before IVIg | |
| 2 | 66 | Female | Yes | 10 | None | 4 mo before IVIg | |
| 3 | 56 | Female | Yes | 10 | None | 17 d before IVIg | |
| 4 | 70 | Male | No | 22 | None | Daily low-dose prednisone | |
| 5 | 35 | Female | No | 23 | None | 3 w before IVIg/steroid | |
| 6 | 45 | Female | No | 22 | None | Daily low-dose prednisone | |
| 7 | 70 | Male | No | 12 | None | Daily 60-mg/mL dose prednisone | |
| 8 | 17 | Female | No | 31 | None | 3 mo before anti-D | |
| 9 | 46 | Female | No | 26 | None | 3 mo before anti-D | |
| 10 | 61 | Female | No | 36 | None | Daily low-dose prednisone | |
| On treatment | |||||||
| Same as patient 9 | 46 | Female | No | 66 | AKR-501 (for 4 mo) | — | |
| Same as patient 10 | 61 | Female | No | 290 | AKR-501 (for 4 mo) | Daily low-dose prednisone | |
| 11 | 53 | Female | Yes | 49 | Romiplostim (for 5 y) | — | |
| 12 | 52 | Male | Yes | 31 | Romiplostim (for 5 y) | — | |
| 13 | 70 | Female | No | 206 | AKR-501 (for 4 mo) | — | |
| 14 | 80 | Female | No | 140 | AKR-501 (for 4 mo) | — | |
| 15 | 51 | Female | No | 280 | Eltrombopag (for 1 y) | — | |
| 16 | 55 | Female | No | 154 | Romiplostim (for 5 y) | — | |
| 17 | 71 | Female | Yes | 70 | Romiplostim (for 5 y) | — |
| Patient no. . | Age, y . | Sex . | Splenectomy . | Platelets, ×109/L . | Thrombopoietic agent (time on treatment) at time of blood sampling/collection . | Previous/ongoing non-TPO-R agonist treatment type at time of blood sampling/collection . | |
|---|---|---|---|---|---|---|---|
| Pretreatment | |||||||
| 1 | 67 | Female | Yes | 29 | None | 5 mo before IVIg | |
| 2 | 66 | Female | Yes | 10 | None | 4 mo before IVIg | |
| 3 | 56 | Female | Yes | 10 | None | 17 d before IVIg | |
| 4 | 70 | Male | No | 22 | None | Daily low-dose prednisone | |
| 5 | 35 | Female | No | 23 | None | 3 w before IVIg/steroid | |
| 6 | 45 | Female | No | 22 | None | Daily low-dose prednisone | |
| 7 | 70 | Male | No | 12 | None | Daily 60-mg/mL dose prednisone | |
| 8 | 17 | Female | No | 31 | None | 3 mo before anti-D | |
| 9 | 46 | Female | No | 26 | None | 3 mo before anti-D | |
| 10 | 61 | Female | No | 36 | None | Daily low-dose prednisone | |
| On treatment | |||||||
| Same as patient 9 | 46 | Female | No | 66 | AKR-501 (for 4 mo) | — | |
| Same as patient 10 | 61 | Female | No | 290 | AKR-501 (for 4 mo) | Daily low-dose prednisone | |
| 11 | 53 | Female | Yes | 49 | Romiplostim (for 5 y) | — | |
| 12 | 52 | Male | Yes | 31 | Romiplostim (for 5 y) | — | |
| 13 | 70 | Female | No | 206 | AKR-501 (for 4 mo) | — | |
| 14 | 80 | Female | No | 140 | AKR-501 (for 4 mo) | — | |
| 15 | 51 | Female | No | 280 | Eltrombopag (for 1 y) | — | |
| 16 | 55 | Female | No | 154 | Romiplostim (for 5 y) | — | |
| 17 | 71 | Female | Yes | 70 | Romiplostim (for 5 y) | — |
All patients “on treatment” were considered responsive to the thrombopoietic agents, where responsiveness is defined as increasing the platelet count from > 30 × 109/L before starting treatment to a count usually > 50 × 109/L while on treatment. The platelet counts correspond to the patients' counts on the day their blood was analyzed for this study. Thus, although patient 12 had a platelet count of 31 × 109/L when we collected blood for our analysis, the common count for this patient while on treatment was > 50 × 109/L. Of a total of 17 patients, 3 patients were receiving daily low-dose (< 3 mg) immunosuppressive prednisone regimen and one on higher dose (60-mg) at the time of their visit, and all except one (patient 10) was in the pretreatment group.
IVIg indicates intravenous immunoglobulin; and —, none.
Proliferation/Treg cell suppression assay
To purify Tregs, CD4+ cells were first enriched by positive selection (Miltenyi Biotec), followed by separation of CD4+CD25hi and CD4+CD25− T cells (94% purity) on a MoFLo cell sorter (Beckman Coulter). For the proliferation assay, CD4+CD25− effector cells (5 × 104) were cultured alone or in combination with CD4+CD25hi Tregs in either duplicates or triplicates at various effector to Treg cell ratios in wells containing irradiated T cell–depleted allogeneic peripheral blood mononuclear cells. On day 5 of culture, 1 μCi of 3H thymidine (PerkinElmer) was added to each well and incubated for an additional 16 hours, and the uptake of labeled thymidine was measured by liquid scintillation (PerkinElmer). Percentage inhibition, which is referred to as Treg-suppressive activity, was determined as 1 − (cpm incorporated in the coculture)/cpm of responder cells alone) × 100.
Surface and Foxp3 staining
To determine the frequency of Tregs, freshly obtained whole blood (150 μL/tube) was first incubated with CD4–peridinin chlorophyll protein and CD25-allophycocyanin (BD Biosciences) before staining for intracellular Foxp3 (clone PCH101 and an isotype control) following the manufacturer's instructions. Cells were analyzed by FACSCanto using Diva software (Version 6.1.3; BD Biosciences).
Cytokine detection
For detection of circulatory TGF-β1 and sCD40L levels, platelet-poor plasma was first prepared using a modified protocol by Lee et al.30 Briefly, blood samples were collected in acid citrate dextrose solution A (BD Vacutainer; BD Biosciences) and first centrifuged at 200g for 15 minutes to remove red blood cells and white blood cells, followed by centrifugation at 750g for 20 minutes to remove platelets and a final centrifugation step at 16 000g for 20 minutes to remove debris. Samples were placed at 37°C for 15 minutes after each centrifugation step, and prostacyclin (5 ng/mL; Sigma-Aldrich) was added before each centrifugation to maintain platelet quiescence. The samples were then defibrinated (1 U/mL thrombin; Sigma-Aldrich) and stored at 80°C. Circulating TGF-β1 levels were determined by the human TGF-β1 Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) following the manufacturer's instructions. To measure total TGF-β1, including the biologically inactive latent TGF-β1 form,31 samples were first acid-activated (with 1N HCl) before assaying. sCD40L was determined by the human ELISA kit by Bender Medsystems (eBioscience).
Intracellular cytokine staining for IL-2 was done on CD4+CD25− sorted T cells stimulated for 5 hours with phorbol myristate acetate (50 ng/mL) and ionomycin (1μM) in the presence of brefeldin A (1 μg/mL;BD Biosciences). Cells were fixed and made permeable with Cytofix/Cytoperm according to the manufacturer's instructions (BD Biosciences). Cells were stained with fluorescein isothiocyanate–conjugated anti–IL-2 and were analyzed on a FACSCanto (BD Biosciences). Flow cytometric data were analyzed with FACS Diva software (Version 6.1.3; BD Biosciences).
Statistical analysis
Statistical analyses were performed using Mann-Whitney U test (platelet counts, Treg frequency, cytokine measurements), nonparametric Spearman correlation (all correlation studies), and 2-way analysis of variance for repeated measurements (Treg assay). Differences were considered significant at P less than .05.
Results
Treg activity improves in patients on treatment with thrombopoietic agents
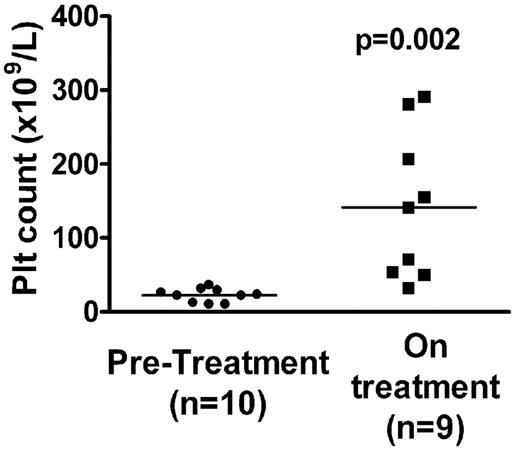
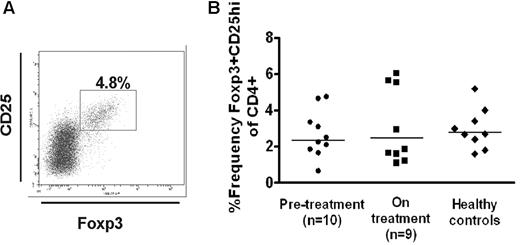
To determine whether Treg frequency/activity is affected after treatment with thrombopoietic agents, we compared the peripheral Treg compartment of a group of chronic ITP patients with platelet counts of less than 40 × 109/L before they went on treatment (n = 10) with that of a second group (n = 9) who had been on treatment with thrombopoietic agents for more than 4 months (Table 1). All but 1 patient (patient 10) in the “on treatment” group was being treated exclusively for ITP with thrombopoietic agents. The “on treatment” group showed an improved platelet count (Figure 1, P = .002). We found no statistically significant differences in the Treg frequencies (Foxp3+CD25hi in the CD4+ population19 ) of patients before treatment and those on treatment (2.7% ± 0.4% vs 3.1% ± 0.6% all P values > .1, Figure 2) and similarly no differences in the levels of Foxp3 expression in the CD4+CD25hi population (data not shown).
Platelet counts in patients before and on treatment with thrombopoietic agents. Platelet counts are shown for the same day that blood was sampled for immunologic analysis. Platelet counts of patients on treatment (■) were statistically higher (P = .002) compared with before treatment (●). Median pretreatment count was 22 × 109/L, interquartile range 11 to 30 × 109/L versus 105 × 109/L, interquartile range 44 to 243 × 109/L on treatment group.
Platelet counts in patients before and on treatment with thrombopoietic agents. Platelet counts are shown for the same day that blood was sampled for immunologic analysis. Platelet counts of patients on treatment (■) were statistically higher (P = .002) compared with before treatment (●). Median pretreatment count was 22 × 109/L, interquartile range 11 to 30 × 109/L versus 105 × 109/L, interquartile range 44 to 243 × 109/L on treatment group.
Frequency of CD25hiFoxp3+ in the patient cohort. (A) Whole blood was stained with anti-CD4 peridinin chlorophyll protein and anti-CD25 allophycocyanin, followed by intracellular staining using phycoerythrin-conjugated anti-Foxp3. The cells were first gated on the CD4+ lymphoid population in peripheral blood mononuclear cells, and the frequency of CD25hiFoxp3+ cells was measured based on the shown gating strategy. (B) Percentage of CD25hiFoxp3+ in the CD4+ population in patients before (●) and on treatment with thrombopoietic agents (■) as well as the normal healthy controls (♦). No statistical differences among the groups were seen.
Frequency of CD25hiFoxp3+ in the patient cohort. (A) Whole blood was stained with anti-CD4 peridinin chlorophyll protein and anti-CD25 allophycocyanin, followed by intracellular staining using phycoerythrin-conjugated anti-Foxp3. The cells were first gated on the CD4+ lymphoid population in peripheral blood mononuclear cells, and the frequency of CD25hiFoxp3+ cells was measured based on the shown gating strategy. (B) Percentage of CD25hiFoxp3+ in the CD4+ population in patients before (●) and on treatment with thrombopoietic agents (■) as well as the normal healthy controls (♦). No statistical differences among the groups were seen.
We next compared the functional activity of CD4+CD25hi subset in patients before and on treatment by purifying and testing them ex vivo using a mixed lymphocyte proliferation assay. CD4+CD25− T cells from patients before and on treatment had similar proliferation capacity (30 825 ± 6518 cpm vs 29 248 ± 4865 cpm, P = .8) and were not significantly different from that of healthy controls (40 387 ± 8054 cpm). Similarly, the sorted CD4+CD25hi from patients in the pretreatment and on treatment groups were equally anergic (4885 ± 899 cpm vs 3436 ± 577 cpm, P = .3), a characteristic feature of in vitro stimulated Tregs.32 However, Treg-suppressive activity, as measured by suppression of proliferation of autologous CD4+CD25− cells from patients on treatment, was significantly higher than the Treg activity of patients in the pretreatment group (Figure 3, at 1:1 ratio, suppressive activity for patients on treatment 63% ± 4% vs 53% ± 7% for pretreatment group and at 1:4, 51% ± 6% vs 35% ± 7% respectively, overall P = .045) but was lower than those of healthy volunteer controls (at 1:1 ratio, 70% ± 2% and at 1:4 ratio, 50% ± 5%). To determine whether the improved Treg activity correlated with altered helper T-cell cytokine secretion pattern, we analyzed the intracellular cytokine expression in the stimulated sorted CD4+ population. It has been reported that peripheral blood mononuclear cells from a majority of patients with chronic ITP when stimulated secrete higher levels of IL-2, possibly as a result of continuously activated autoimmune responses.5 We found a significant negative correlation between levels of IL-2 expression in stimulated, sorted CD4+ T cells and Treg-suppressive activity (Figure 4, r = −0.47, P = .04), suggesting that improved Treg activity may be linked to dampening of immune responses.
Circulating Treg-suppressive activity in patients before and on treatment with thrombopoietic agents. Suppression of proliferation by CD4+CD25hi Treg was analyzed in patients before treatment (n = 10) and in patients on treatment with thrombopoietic agents (n = 9) as well as normal healthy controls (n = 9). Sorted populations of CD4+CD25− T cells were stimulated with plate-bound 0.1 μg/mL anti-CD3 antibodies and allogeneic accessory cells, alone or cocultured at various ratios (solid lines indicate 1:1; and dotted lines, 1:4) with autologous sorted CD4+CD25hi cells, and mean inhibition was calculated as described in “Proliferation/Treg cell suppression assay.” Although this is a cross-sectional study with different patients in the pretreatment and on treatment groups, we have joined the pretreatment and on treatment data points by a line (solid or dotted) to highlight the difference in the suppressive activity between the 2 groups. Suppression measured at a 1:1 ratio of CD4+CD25− to CD4+CD25hi in patients before treatment was 53% ± 7% but was higher in patients on treatment (63% ± 4%). Similarly, at 1:4, the pretreatment group had a lower suppressive activity (35% ± 7%) compared with on treatment group of 51% ± 6% (overall P = .045). For comparison, the healthy volunteer controls' suppressive activity at 1:1 ratio (70% ± 2%) and at 1:4 ratio (50% ± 5%) is also indicated. The raw proliferation data are shown in supplemental Figure 2.
Circulating Treg-suppressive activity in patients before and on treatment with thrombopoietic agents. Suppression of proliferation by CD4+CD25hi Treg was analyzed in patients before treatment (n = 10) and in patients on treatment with thrombopoietic agents (n = 9) as well as normal healthy controls (n = 9). Sorted populations of CD4+CD25− T cells were stimulated with plate-bound 0.1 μg/mL anti-CD3 antibodies and allogeneic accessory cells, alone or cocultured at various ratios (solid lines indicate 1:1; and dotted lines, 1:4) with autologous sorted CD4+CD25hi cells, and mean inhibition was calculated as described in “Proliferation/Treg cell suppression assay.” Although this is a cross-sectional study with different patients in the pretreatment and on treatment groups, we have joined the pretreatment and on treatment data points by a line (solid or dotted) to highlight the difference in the suppressive activity between the 2 groups. Suppression measured at a 1:1 ratio of CD4+CD25− to CD4+CD25hi in patients before treatment was 53% ± 7% but was higher in patients on treatment (63% ± 4%). Similarly, at 1:4, the pretreatment group had a lower suppressive activity (35% ± 7%) compared with on treatment group of 51% ± 6% (overall P = .045). For comparison, the healthy volunteer controls' suppressive activity at 1:1 ratio (70% ± 2%) and at 1:4 ratio (50% ± 5%) is also indicated. The raw proliferation data are shown in supplemental Figure 2.
Correlative analysis of Treg functional activity and frequency of IL-2–expressing sorted CD4+ T cells. Sorted CD4+CD25− T cells from the patient cohort were stimulated with phorbol myristate acetate and ionomycin for 5 hours in the presence of protein transport inhibitor, and intracellular expression of IL-2 was measured by flow cytometry. The frequency of IL-2-expressing CD4+ cells negatively correlated with the patients' Treg-suppressive activity at 1:1 ratio of CD4+CD25− and CD4+CD25hi (r = −0.47, P = .04).
Correlative analysis of Treg functional activity and frequency of IL-2–expressing sorted CD4+ T cells. Sorted CD4+CD25− T cells from the patient cohort were stimulated with phorbol myristate acetate and ionomycin for 5 hours in the presence of protein transport inhibitor, and intracellular expression of IL-2 was measured by flow cytometry. The frequency of IL-2-expressing CD4+ cells negatively correlated with the patients' Treg-suppressive activity at 1:1 ratio of CD4+CD25− and CD4+CD25hi (r = −0.47, P = .04).
Circulating plasma levels of TGF-β1 and sCD40L
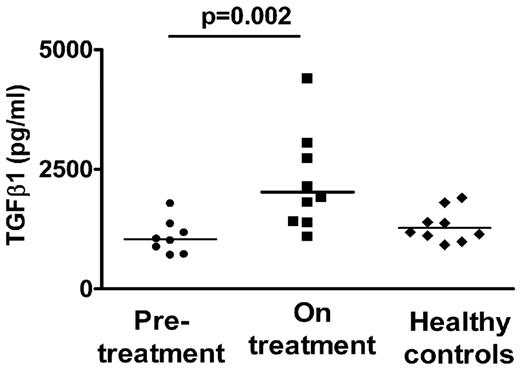
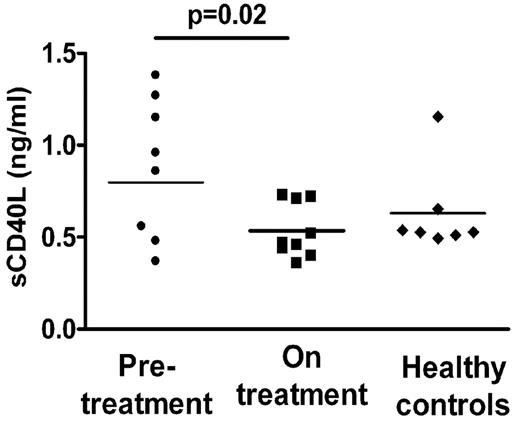
TGF-β1 is primarily considered an anti-inflammatory cytokine, promoting Treg development and function and inhibiting Th1 and Th2 development as well as B-cell proliferation and antibody production.33 A number of studies have indicated that ITP patients have low circulating TGF-β1 levels, which increase as platelet counts improve after various treatments.34,–36 We therefore measured total circulating TGF-β1 in some of our patient cohort using the platelet-poor fraction of the plasma to avoid potential in vitro platelet activation/degranulation. We found that circulating TGF-β1 levels strongly correlated with platelet counts in our patient cohort (Figure 5, r = 0.8, P = .0002) with higher levels of TGF-β1 in the patient group on treatment compared with the pretreatment group (Figure 6, 2211 ± 345 pg/mL vs 1083 ± 127 pg/mL, P = .02). Concurrently, we also tested levels of circulating sCD40L (Figure 7), a proinflammatory cytokine in the platelet-poor plasma fraction in our patient cohort, and found a decrease in the levels in patients on treatment (0.88 ± 0.13 ng/mL vs 0.53 ± 0.05 ng/mL, P = .02) as has previously been reported in treated patients with ITP.11
Correlative study of platelet counts and TGF-β1 levels. Circulating total TGF-β1 levels of the patient cohort from Table 1 were measured by ELISA using platelet-poor plasma. Correlative analysis indicated a strong positive association between the patients' platelet counts and TGF-β1 levels (r = 0.8, P = .0002).
Correlative study of platelet counts and TGF-β1 levels. Circulating total TGF-β1 levels of the patient cohort from Table 1 were measured by ELISA using platelet-poor plasma. Correlative analysis indicated a strong positive association between the patients' platelet counts and TGF-β1 levels (r = 0.8, P = .0002).
Circulating TGF-β1 levels in patients before and on treatment with thrombopoietic agents. Plasma levels of TGF-β1 were significantly increased in patients on treatment (■) compared with before treatment (●, P = .002, using the rank order Mann-Whitney test). As comparison, levels of TGF-β1 in normal healthy controls are shown (♦).
Circulating TGF-β1 levels in patients before and on treatment with thrombopoietic agents. Plasma levels of TGF-β1 were significantly increased in patients on treatment (■) compared with before treatment (●, P = .002, using the rank order Mann-Whitney test). As comparison, levels of TGF-β1 in normal healthy controls are shown (♦).
sCD40L levels in platelet-poor plasma of patient cohort. Circulating levels of sCD40L in platelet-poor plasma were significantly lower in patients on treatment (■) compared with pretreatment (●, P = .02). As comparison, levels of sCD40L in normal healthy controls are shown (♦).
sCD40L levels in platelet-poor plasma of patient cohort. Circulating levels of sCD40L in platelet-poor plasma were significantly lower in patients on treatment (■) compared with pretreatment (●, P = .02). As comparison, levels of sCD40L in normal healthy controls are shown (♦).
Discussion
We have found in a small cross-sectional study improved in vitro Treg function in chronic ITP patients who have been on thrombopoietic agents, suggesting, for the first time, that these agents may possess immunomodulatory activity in addition to their profound effects on platelet counts. The increase in Treg-suppressive activity correlated with a reduction in effector T helper functions as manifested by a negative association between Treg activity and the frequency of IL-2–expressing CD4+ T cells. Moreover, patients on treatment had increased circulating TGF-β1 levels but decreased sCD40L levels, both suggesting a reduction in the overall inflammatory state. Whether the increase in Treg activity in patients on treatment is a cause or consequence of decrease in the inflammatory state remains to be determined by future longitudinal studies.
Because TPO receptor expression is largely restricted to the megakaryocytic compartment,37 it is unlikely that the thrombopoietic agents directly interact with the Tregs to improve their activity. Instead, our hypothesis is that the effect of thrombopoietic agents on Tregs is mediated indirectly by TGF-β1 released by platelets and/or megakaryocytes. In support of this latter possibility, we found a strong association between platelet counts and circulating TGF-β1 levels. Platelets are a rich source of TGF-β1, containing 40 to 100 times more than other cell types,38 and they release TGF-β1 after activation/degranulation.38 The cause of elevated TGF-β1 levels in patients on thrombopoietic agents is not known, but an artifactual platelet activation during venipuncture only in one patient group, and not in healthy controls who have equal or higher platelet counts, seems unlikely. Moreover, we find decreased levels of sCD40L, known to be abundant in platelets and released after platelet activation39 in the same plasma samples in patients on TPO-R agonists, further arguing against an artifactual platelet activation during venipuncture. These data further support a previous report40 that platelets are not activated in patients on thrombopoietic agents. The reason why circulatory sCD40L, which are mostly platelet-derived, are higher in patients in our pretreatment group as well as in patients with low platelet counts11 is not known, but it may be that sCD40L is derived from a nonplatelet source (eg, T cells)41 in patients who have low platelet counts. In addition, the mechanisms that cause the release of sCD40L, but not TGF-β1, in patients with low platelet counts remain to be fully determined, but it is possible that these cytokines may belong to different platelet granule sorting/release pathways.42
TGF-β1 may also be released by (increased numbers of) megakayocytes.43 It is possible that with increased platelet turnover in patients on thrombopoietic agents, there is increased platelet clearance with concomitant release of TGF-β1. Interestingly, ITP patients with improved platelet counts after treatment with immunosuppressive drugs, high-dose dexamethasone, intravenous immunoglobulin, or splenectomy were also shown to have increased TGF-β1 levels,34,–36 although direct correlation studies between TGF-β1 levels and platelet counts were not reported. These latter treatments, when effective, are thought to prevent or minimize platelet destruction, which is in contrast to the effect of thrombopoietic agents that increase platelet turnover. Future studies are needed to determine the mechanisms by which TGF-β1 levels are increased in patients on thrombopoietic agents and how these mechanisms may differ, if at all, in patients who are on treatments that interfere with platelet destruction. Alternatively, it may also be that, as platelet counts improve, there is altered processing/presentation of platelet antigens by the antigen-presenting cells, resulting in Treg normalization.
TGF-β1 can induce Foxp3-negative T cells and convert them to Foxp3+ Tregs.44 Although we did not find any correlation between TGF-β1 levels and the frequency of Foxp3+ cells in the CD4+CD25hi population, we did find a positive correlation between TGF-β1 levels and the frequency of total CD4+Foxp3+ cells (r = 0.6, P = .02, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These latter cells consisted of CD4+CD25hi Treg population with known regulatory function as well as the CD4+CD25med and CD4+CD25− populations, whose regulatory role remains questionable.45,46 Longitudinal studies are needed to determine whether TGF-β1 levels correlate with induction of Foxp3+ T cells in various CD4+CD25hi/med/− populations and, more importantly, if these Foxp3-expressing CD4+CD25med and CD4+CD25− subpopulations possess regulatory activity in ITP patients on treatment.
TGF-β1 has also been shown to be essential for maintenance of Treg functions.44 Specifically, CD4+CD25+ Treg from transgenic mice overexpressing a truncated version of the TGF-β type II receptor that acts as a dominant negative mutant receptor had decreased in vivo suppressive capacity, indicating that TGF-β1 signaling is essential to maintain Treg-suppressive function.47 We were unable to find a direct correlation between Treg-suppressive activity and TGF-β1 levels. However, in 2 cases (patients 9 and 10, Table 1), we were able to perform analysis before and on treatment on the same patients. With patient 9 whose platelet count was 26 × 109/L before treatment and increased to 66 × 109/L on the drug, we found a 20% increase in peripheral TGF-β1 levels and a concomitant 20% improvement in Treg activity, whereas in patient 10, with platelet counts of 36 × 109/L before treatment to 290 × 109/L on treatment, TGF-β1 levels and Treg activity both increased by 30%. Because our study had a cross-sectional design, longer-term longitudinal studies are required to validate the clinical significance of the elevation of Treg activity observed in patients on treatment with thrombopoietic agents and to further explore the role of platelets and its association with Tregs in ITP. The recent development of a mouse model of ITP48 is likely to be helpful in addressing some of these questions.
It is our prediction that antigen-specific Treg responses are increased in patients on treatment in parallel to polyclonal Treg activity, but these studies have not yet been performed. In the study by Kuter et al,25 only approximately 10% of the ITP patients treated with a thrombopoietic agent for 6 months did not relapse in the 12 weeks of follow-up. What fraction of patients will relapse when longer (ie, years instead of months) therapy is discontinued remains unexplored and needs further study. Of note, 5 of 9 patients in the on treatment group had been taking the thrombopoietic agents for more than or equal to 1 year (Table 1). It is possible that, because functional Tregs are already induced in these patients, treatment regimens optimized to fully restore platelet counts will gradually lead to establishment of stable Treg populations to achieve long-term immune tolerance.
In conclusion, we have found an improved in vitro Treg activity with a concomitant decrease in effector T helper functions in patients on treatment with TPO-R agonists. Furthermore, patients on treatment had increased circulating TGF-β1 levels but decreased sCD40L levels, indicating a reduction in the overall inflammatory state. Although the data need to be validated in longitudinal study, our current study suggests that thrombopoietic agents, which have already been shown to be highly effective in the treatment of ITP, may also be pro-tolerogenic, too.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Zeeshan Hafeez and Jared Levan, Jessica Cruz, and Greg Lallos (Weill Cornell Medical College) for help with coordinating some of the patient recruitment and clinical data analysis. We thank Dr Beau Mitchell (NYBC) for helpful discussions.
This work was supported in part by National Heart, Lung, and Blood Institute grant HL096497-01 (K.Y.).
National Institutes of Health
Authorship
Contribution: W.B., S.H., and W.H. performed research and analyzed and interpreted data; M.K. and N.B. recruited patients and analyzed data; J.B.B. designed the research, selected and recruited patients, and wrote the paper; and K.Y. designed, directed, and performed research and wrote the paper.
Conflict-of-interest disclosure: J.B.B. received clinical research support from the following companies: Amgen, Camgene, GlaxoSmithKline, Genzyme, Immunomedics, Ligand, Eisai Inc, Shionogi, and Sysmex. He also participates in the France Foundation speaker's bureau program. His family owns stock in Amgen and GlaxoSmithKline. He has participated in Advisory Boards for Amgen, GlaxoSmithKline, Ligand, Shionogi, and Eisai. The remaining authors declare no competing financial interests.
Correspondence: Karina Yazdanbakhsh, Laboratory of Complement Biology, New York Blood Center, 310 E 67th St, New York, NY 10065; e-mail: kyazdanbakhsh@nybloodcenter.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal