Antibody-mediated pure red cell aplasia is a rare but serious event resulting from the induction of neutralizing erythropoietin (Epo)–specific antibodies provoked by treatment with recombinant Epo. Because of the crucial role of CD4 T cells in humoral response, we have quantified the number of Epo-specific CD4 T cells in the blood of normal donors by in vitro stimulation. An important repertoire of preexisting Epo-specific T cells was observed in almost half of the donors, comparable with that of non–self-proteins. This observation suggests that, at the steady state, endogenous Epo weakly contributes to tolerance induction and may be ignored by the immune system. As a result, circulating Epo-specific CD4 T cells could be prone to be activated by altered batches of Epo, providing them with costimulatory signals. Our data also highlight the relevance of T-cell assays performed with normal donors to evaluate the potential immunogenicity of therapeutic proteins.
Introduction
Millions of patients have been injected with recombinant forms of human erythropoietin (Epo) to treat anemia. In rare cases, these injections provoke a humoral response specific to Epo, leading to pure red-cell aplasia, a serious hematologic disorder characterized by a deficiency in mature erythroid progenitors.1 By the end of the 1990s, a sudden increase in the frequency of pure red-cell aplasia was associated with a change in the administration route of Epo and the removal of human serum albumin (HSA) from its formulation.1 Intensive analytical characterization of HSA-free batches of Epo was performed to understand this increase in immunogenicity. Association of Epo with polysorbate micelles,2 formation of aggregates,3 and the presence of leachates originating from the uncoated rubber plunger4 were proposed as the main causes of immunogenicity. After introduction of a coated rubber plunger, contraindication of subcutaneous injection, and improvement of storage conditions, the rate of pure red-cell aplasia rapidly reached the initial background level. Safety of the products appeared now to be under control, but the precise reasons for the sudden immunogenicity of some HSA-free batches of Epo are still a source of controversy.
Several lines of evidence indicate that antibody response to therapeutic proteins is T cell dependent.5,–7 CD4 T-cell activation is known to result from at least 2 different signals.8,9 The first signal is provided through T-cell receptor recognition of the antigenic peptides presented by human leukocyte antigen (HLA) class II molecules, whereas the second signal is delivered by costimulation molecules to the T cells.8 As immature dendritic cells (DCs) are relatively inefficient at providing the second signal, DC maturation contributes to initiation of the immune response.10 Maturation is provoked by bacterial and viral components, such as lipopolysaccharide and cytosine-phosphate diester-guanine through interactions with Toll-like receptors,11 and by particulate moieties, such as crystals and microparticles through interactions with inflammasome.12 DC maturation was also shown to be induced by synthetic compounds, such as imiquimod,13 degradation products of the stabilizer polysorbate used in HSA-free Epo batches,14 and aggregated forms of polypeptides.15,16 Although it has not been formerly demonstrated, chemical alterations of HSA-free batches of Epo might therefore have provided costimulatory signals. However, signaling by innate receptors does not fully explain the sudden immunogenicity of Epo. In the absence of preexisting antigen-specific CD4 T cells, antigen injection cannot lead to immune response, even in the presence of adjuvant.17,18 An important issue for the understanding of Epo immunogenicity is therefore to evaluate the repertoire of preexisting Epo-specific CD4 T cells.
In this work, using in vitro repeated stimulations of Epo-specific CD4 T cells, we observed a surprisingly high frequency of precursors of Epo-specific CD4 T cells in almost half of the donors.
Methods
Proteins
Keyhole limpet hemocyanin (KLH) and glutathione S-transferase were from Thermo-Fisher Scientific. Epo (Eprex, Janssen-cilag) was a kind gift from Dr J.-L. Prugnaud (Saint-Antoine Hospital, Paris, France). Ovalbumin was from Sigma-Aldrich, and human insulin was from Clinisciences. Antithrombin III and α-antitrypsin were from Calbiochem.
Generation of KLH- and Epo-specific CD4 T-cell lines from healthy donors
Peripheral blood mononuclear cells were collected from healthy donors (EFS) and genotyped using the Gold SSP DRB1 typing kit (Invitrogen). Coculture of antigen-loaded DCs and CD4 T lymphocytes was performed as described previously,19 except that immature DCs were used to load the antigens (S.D., G.R., B.M., manuscript submitted). T-cell line specificity was investigated by interferon-γ ELISpot assays19 at day 21 (S.D., G.R., B.M., manuscript submitted). A positive value was assigned to culture wells with a spot count that was 2-fold higher in presence of the protein compared with control cells without the protein, with a minimal difference of 30 spots and a positivity in a statistical Student t test evaluation (P < .05). CD4+ T-cell precursor frequency was estimated using the Poisson distribution according to the following formula: Frequency = −Ln(number of negative wells/total number of wells tested)/(number of CD4+ T cells/well).
Results and discussion
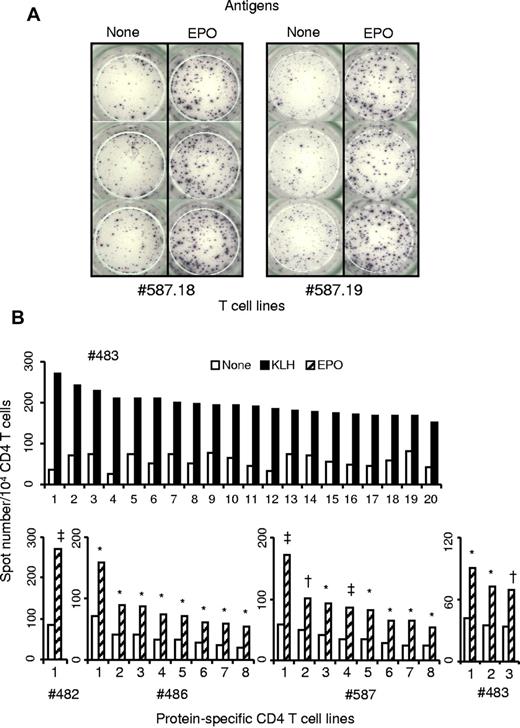
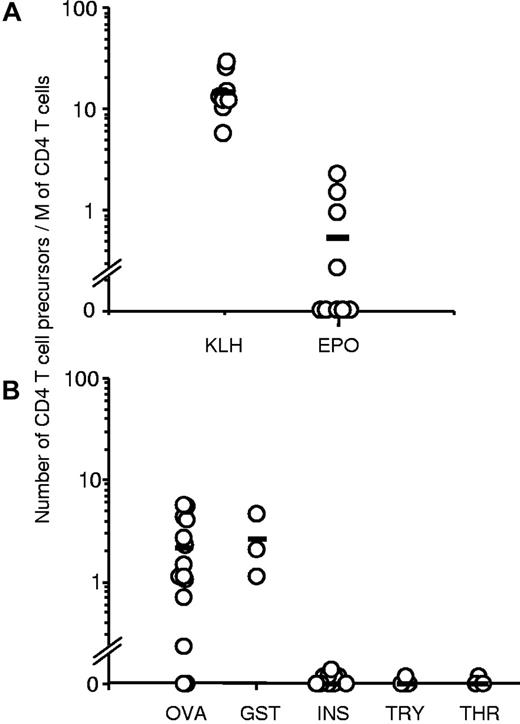
Purified CD4 T cells collected from 9 healthy donors were seeded in 20 wells of culture plates and weekly stimulated by DCs loaded with Epo or the positive control KLH. Each independent T-cell line (expanded and unsorted T cells present in a well) was evaluated for their content in protein-specific T cells by interferon-γ ELISpot assay. Examples of T-cell lines specific for Epo are shown in Figure 1A. The healthy donors exhibited multiple HLA-DR allotypes, including most of the preponderant alleles present in Europe (supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For all the donors, a high proportion of KLH-specific T-cell lines was found, varying from 66% to 100%, as illustrated for donor 483 (Figure 2A). Eight independent Epo-specific T-cell lines were isolated from donors 486 and 587, one from donor 482, and 3 from donor 483 (Figure 1B). The 5 other donors remained nonresponders to Epo. We then used these results to calculate the frequency of preexisting Epo-specific CD4 T cells. The initial distribution of the protein-specific T cells followed a Poisson distribution as we showed previously (S.D., G.R., B.M., manuscript submitted). The mean number of KLH-specific T-cell precursors was near 15 cells per 106 cells (Figure 2A). This number was underestimated as 5 donors produced a maximum of 100% of T-cell lines specific for KLH. For Epo, the 4 responding donors exhibited a frequency of preexisting CD4 T cells varying from 0.27 to 2.2 cells per million. The mean of all the donors, including the nonresponding donors is 0.53 cells per million. Interestingly, 3 of the 4 responding donors possessed an Epo-specific repertoire closed to 1 cell per million, whereas the non–self-proteins ovalbumin and glutathione S-transferase reached a mean level of 2.16 and 2.6, respectively. In contrast to Epo, other self-proteins, such as human insulin, antithrombin III, and α-antitrypsin, did not show any in vitro T-cell response.
Specificity of protein-specific CD4 T-cell lines raised against KLH or human Epo. Expanded unsorted CD4 T cells were obtained after repeated weekly stimulations of CD4 T lymphocytes purified from healthy donors by autologous mature DCs loaded with KLH or Epo. Reactivity of all the CD4 T-cell lines was assessed by interferon-γ ELISpot assay in the presence and in absence of KLH or Epo. Briefly 104 CD4 T cells were incubated in triplicate with 5 × 103 autologous unloaded DCs or DCs loaded with the appropriate antigen. Spot staining was done after 24-hour incubation at 37°C. (A) Examples of ELISpot wells from 2 Epo-specific T-cell lines. (B) KLH-specific T-cell lines of donor #483 and Epo-specific T-cell lines derived in this study. *P < .05. †P < .005. ‡P < .0005.
Specificity of protein-specific CD4 T-cell lines raised against KLH or human Epo. Expanded unsorted CD4 T cells were obtained after repeated weekly stimulations of CD4 T lymphocytes purified from healthy donors by autologous mature DCs loaded with KLH or Epo. Reactivity of all the CD4 T-cell lines was assessed by interferon-γ ELISpot assay in the presence and in absence of KLH or Epo. Briefly 104 CD4 T cells were incubated in triplicate with 5 × 103 autologous unloaded DCs or DCs loaded with the appropriate antigen. Spot staining was done after 24-hour incubation at 37°C. (A) Examples of ELISpot wells from 2 Epo-specific T-cell lines. (B) KLH-specific T-cell lines of donor #483 and Epo-specific T-cell lines derived in this study. *P < .05. †P < .005. ‡P < .0005.
Frequencies of preexisting CD4 T cells specific to KLH and Epo in the blood of normal donors. The estimate of protein-specific T-cell precursors was done on the basis of the percentage of negative wells and the number of distributed CD4 T cells according to the following formula of the Poisson law: frequency = −Ln(number of negative wells/total number of wells tested)/(number of CD4+ T cells/well). (A) Number of KLH- and human Epo-specific CD4 T cells was estimated from 9 HLA-typed normal donors (top panel). (B) Estimates were performed from 14 donors for ovalbumin (OVA), 3 for glutathione S-transferase (GST), 11 for insulin (INS), 4 for α-antitrypsin (TRY), and 3 for antithrombin III (THR).
Frequencies of preexisting CD4 T cells specific to KLH and Epo in the blood of normal donors. The estimate of protein-specific T-cell precursors was done on the basis of the percentage of negative wells and the number of distributed CD4 T cells according to the following formula of the Poisson law: frequency = −Ln(number of negative wells/total number of wells tested)/(number of CD4+ T cells/well). (A) Number of KLH- and human Epo-specific CD4 T cells was estimated from 9 HLA-typed normal donors (top panel). (B) Estimates were performed from 14 donors for ovalbumin (OVA), 3 for glutathione S-transferase (GST), 11 for insulin (INS), 4 for α-antitrypsin (TRY), and 3 for antithrombin III (THR).
We have therefore quantified in HLA-DR–unrelated normal donors the frequency of preexisting Epo-specific CD4 T lymphocytes that, remarkably, is in agreement with the T-cell epitope mapping of Epo.20 Although the objective of this latter work was not to quantify the Epo-specific repertoire, the authors derived Epo-specific T-cell lines in 5 of 7 donors.20 The values we observed for KLH were also in agreement with recently published data.21 Human proteins are thought to be the target of an important immune tolerance, but our work strongly suggested that endogenous Epo is at least partially ignored by the immune system. Immune ignorance is defined by the presence of self-reactive T cells that may remain functionally unresponsive because of a lack of appropriate T-cell activation.22 We proposed that endogenous of Epo did not drastically limit the size of its T-cell repertoire by the thymic and peripheral selection as it has been already demonstrated in transgenic mice.23,24 Because of the presence of Epo-specific T cells in the blood, costimulation signals provided by the alteration of HSA-free Epo batches2,–4 could be sufficient to initiate a T-cell response and the subsequent production of neutralizing antibodies. Our data illustrate the limit of sequence humanization of therapeutic proteins to prevent immunogenicity and highlight the relevance of in vitro quantitative T-cell amplification assays to assess the immunogenicity potential of therapeutic proteins.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J.-L. Prugnaud for the kind gift of Epo and Pr M. Pallardy for critical reading of the manuscript.
This work was supported by Protéus and the CEA.
Authorship
Contribution: S.D. designed and performed research, analyzed and interpreted the data, performed statistical analysis, and wrote the manuscript; G.R. designed research and wrote the manuscript; and B.M. designed research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: S.D. and G.R. are employed by the biotechnology company Proteus. B.M. is scientific advisor of Proteus.
Correspondence: Bernard Maillere, CEA, Institute of Biology and Technologies, SIMOPRO, Bat 152, Gif-sur-Yvette, F-91191, France; e-mail: bernard.maillere@cea.fr; or Stéphanie Delluc, Protéus 70, allée Graham Bell, Parc Georges Besse, Nimes, F-30000, France; e-mail: sdelluc@proteus.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal