Abstract
Abstract 960
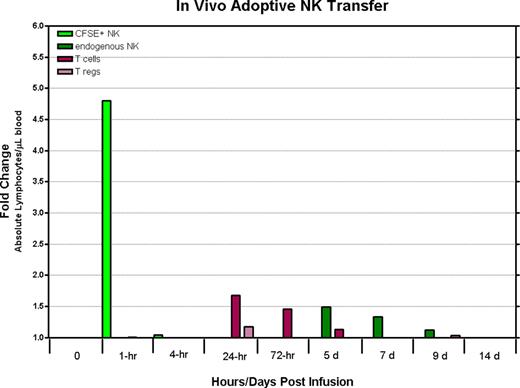
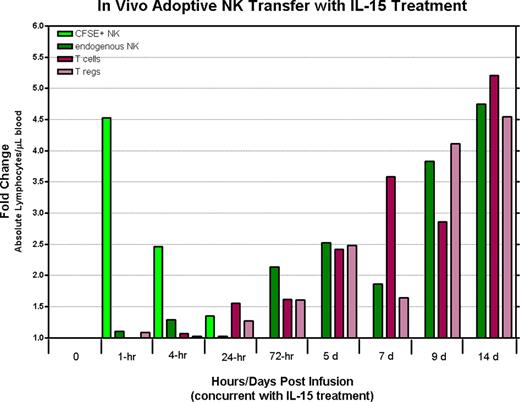
Immunotherapy using natural killer (NK) cells is currently being explored as a treatment option for patients with advanced malignant diseases. Although pilot clinical trials have shown adoptive NK cell transfer can result in tumor regression in humans with cancer, additional insight from animal models is needed to optimize methods to enhance the function and in vivo persistence of these adoptively infused lymphocytes. In contrast to mice, rhesus macaques have orthologues to most of the human MHC class I and II genes and possess NK cells expressing KIRs that are phenotypically and functionally similar to human NK cells, thus providing an excellent model to evaluate adoptive NK cell therapy. To characterize their in vivo longevity and tissue trafficking following adoptive infusion, we developed a method to expand large numbers of rhesus NK cells in vitro. NK cells enriched from peripheral blood mononuclear cells by depleting CD3+ cells using immunomagnetic beads were expanded in vitro with autologous plasma and a human EBV-LCL feeder cell line using culture conditions identical to those used for human NK cell expansion. Expanded rhesus NK cells were both phenotypically and genotypically similar to their human counterparts; NK cell cultures expanded up to 1000 fold within 2–3 weeks, were greater than 99% CD3 negative, and had a large proportion of CD16/CD56 double positive cells. In addition, expanded NK cells up-regulated receptors involved in tumor killing, including NKG2D, Granzyme B, TRAIL and Fas-ligand and were highly cytotoxic to K562 cells. Adoptive transfer of (3.2×107 – 1×108) CFSE-labeled ex vivo expanded rhesus NK cells has been well tolerated without any overt toxicities noted to date. Remarkably, despite the infusion of large cell numbers, CFSE labeled NK cells were detectable in the peripheral blood, lymph nodes, and bone marrow compartments at very low levels for only a few hours following infusion. Combining adoptive transfer of ex vivo expanded NK cells with IL-15 administration (rhesus recombinant IL-15 10 ug/kg s.c. × 5 days) resulted in only a minimal and transient 24 hour increase in the number of detectable CFSE labeled NK cells in the circulation and bone marrow. Although IL-15 administration did not substantially expand the number of circulating CFSE labeled NK cells that were adoptively transferred, it did result in a substantial increase in circulating numbers of endogenous NK and T-cells (4.74 fold and 5.2 fold increase in CD3-/CD56+ NK cells and CD3+ T-cells respectively). Surprisingly, IL-15 administration also resulted in a significant expansion of circulating T-regs (CD4-/CD25+/CD127Dim/FOXP3 +) which have previously been shown to suppress NK cell effector function in vitro and vivo; T-cells with a regulatory phenotype expanded 4.54 fold. Expansion of circulating T-regs occurred both when IL-15 was administered alone or in conjunction with adoptive NK cell transfer.
IL-15 administration in macaques at the doses used in this study did not expand circulating numbers of adoptively transferred ex-vivo expanded NK cells, although it did significantly expand the numbers of circulating endogeneous NK cells. Remarkably, IL-15 administration was also associated with a significant expansion of T-cells with a regulatory phenotype. We are currently evaluating whether lympho-depletion followed by adoptive NK cell transfer can be used as a method to prevent the expansion of T-regs associated with IL-15 administration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal