Abstract
Abstract 835
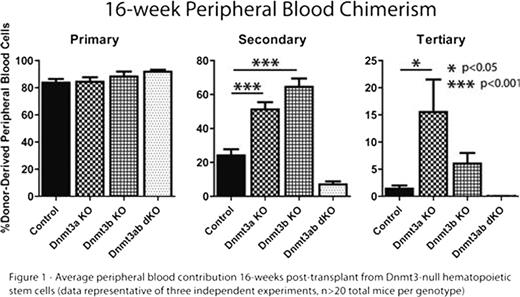
DNA methylation is one of the major epigenetic modifications in the vertebrate genome and is catalyzed by the DNA methyltransferase enzymes Dnmt1, Dnmt3a and Dnmt3b. We observed a dynamic expression profile of Dnmt3a and Dnmt3b in the hematopoietic system with both enzymes expressed at exponentially higher levels in hematopoietic stem cells (HSCs) compared to progenitors and differentiated cells and hypothesized that some of the unique characteristics of HSCs were epigenetically regulated by Dnmt3a and Dnmt3b. To study this, we crossed Dnmt3a and -3b conditional knock-out (KO) mice to Mx1-cre mice to generate inducible single- and double-KO (dKO) mice. We performed competitive transplantation of HSCs (side-population+c-Kit+Lineage-Sca-1+ = SPKLS) from these mice along with wild-type whole bone marrow competitor and induced deletion of the Dnmt3's in the donor cells by sequential pIpC injections in the wild-type recipients. No dramatic differences were observed in primary recipients in the absence of other hematopoietic perturbation, however when we re-transplanted Dnmt3a- and Dnmt3b-KO HSCs into secondary recipients, they exhibited surprisingly high peripheral blood reconstitution compared to control HSCs (>4-fold increase in engraftment). This was reflected in the bone marrow of these mice with a corresponding >4-fold expansion of the HSC pool (phenotypically defined by any of SPKLS; CD34-Flk2-KLS; CD150+CD48-KLS) with virtually all of these cells being derived from the Dnmt3a- and Dnmt3b-KO donor HSCs. Consistent with a previous study, we observed a decline in functional output of Dnmt3a/3b-dKO HSCs in secondary transplants in terms of peripheral blood chimerism, but surprisingly these mice also exhibited a modest expansion of the HSC pool (∼2-fold), the majority of which were derived from donor Dnmt3a/3b-dKO HSCs. In subsequent tertiary and quaternary transplantation, Dnmt3 single-KO HSCs remained highly superior in peripheral blood engraftment capacity relative to control HSCs and Dnmt3a/3b-dKO HSCs (Figure 1), although the expansion of the HSC pool in all Dnmt3-KOs continued to varying degrees. This enhanced HSC activity appears to be a cell autonomous mechanism as purified Dnmt3-KO SPKLS cells from transplanted mice have much greater hematopoietic colony forming potential in vitro compared to control HSCs on a per cell basis. However the observed HSC expansion does not appear attributable to either enhanced proliferation of Dnmt3-KO HSCs or more resistance to apoptosis. The serially-transplanted Dnmt3-KO HSCs are not overtly transformed, in that the levels of differentiated blood cells are still normal and the mice appear to be healthy. This may be akin to a pre-malignant state seen in human myelodysplastic syndrome. We have performed microarray expression profiling of serially-transplanted Dnmt3-KO HSCs and identified several candidate genes which are currently being investigated as the mechanism for HSC expansion. Our data suggest ablation of de novo DNA methylation in HSCs uncouples normal self-renewal and differentiation. These studies present further evidence for the contribution of epigenetic regulation to stem cell activity and provide a tantalizing link between potential aberrant methylation in HSCs contributing to leukemic transformation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal