Abstract
Abstract 759
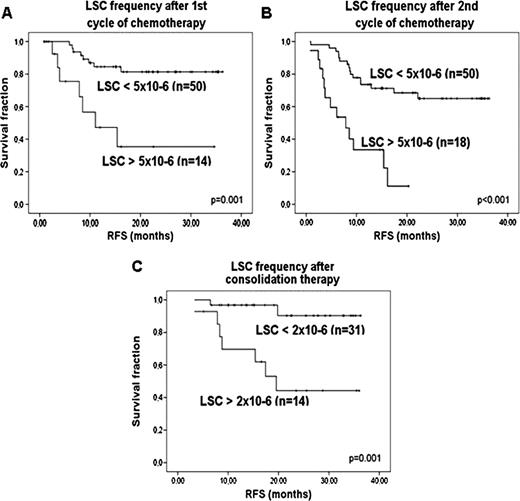
Detecting leukemia initiating cells or leukemic stem cells (LSCs) in acute myeloid leukemia (AML) is a challenge. AML is a heterogeneous disease and since the first studies on LSCs, different immunophenotypic cell compartments have been reported to contain LSCs. For CD34 positive (CD34+) patients, the most important LSC compartment is most likely CD34+CD38-. However, both LSCs and normal hematopoietic stem cells (HSCs) reside in this compartment. Therefore, LSC detection requires the ability to accurately discriminate between LSCs and HSCs. Previous research showed that LSCs, in contrast to HSCs, expressed aberrant markers (AM, including lymphoid lineage markers and the LSC marker CLL-1). Although AM- cells are often presumed to be normal HSCs, AM expression can be absent or very weak on LSCs and AM- cells can therefore be malignant too. We previously described that in 30% of the patients, besides the AM expression, additional FACS parameter (forward scatter (FSC) and sideward scatter (SSC) and/or CD34 expression levels) could also be used to detect LSCs (Terwijn et al., ASH 2009 abstract 399). To prove that the CD34+CD38- fraction contains both normal and malignant SCs, and in addition, to prove that AM- cells can be malignant too, we studied molecular aberrancies (3 cases of FLT3-ITD positive AML) in the relevant cell fractions. 1: CD34+CD38-AM+ cells proved to be FLT3-ITD positive in all three cases. In addition, these cells initiated leukemic engraftment in NOD-SCID IL-2Rγ -/- mice in 2/2 cases. 2: The CD34+CD38-AM- cells with a FSC/SSC low or CD34 high character were FLT3-ITD negative in 3/3 cases and initiated multilineage engraftment in mice in 2/2 cases, indicating that these are HSCs. 3: The CD34+CD38-AM- cells with a FSC/SSC high or CD34 low phenotype were FLT3-ITD positive in 2/2 cases and therefore malignant, indicating that AM expression alone is not always sufficient in discriminating HSCs from LSCs. Since therapy surviving LSCs are thought to be responsible for relapses, we hypothesized that LSC frequency, as a fraction of total WBC, detected in remission bone marrow (BM) would provide prognostic information. Using AM as a primary gating strategy and, when applicable, FSC/SSC and/or CD34 expression, as a secondary gating step, LSC analysis was performed in 112 CD34+ AML patients (<60 years). In 76/112 patients, LSCs were detected using the most specific AM (CLL-1 (n=45), CD7 (n=19), CD11b (n=4), CD56 (n=3), CD22 (n=2), CD19 (n=2) and CD36 (n=1)) as a primary gating strategy. In 20 of these patients, a secondary gating step in FSC/SCC (n=16) and CD34 expression (n=4) refined the malignant population. In 36/112 patients, no AM was expressed on the CD34+CD38- cells, but in 25 cases, FSC/SSC could be used to gate the malignant fraction, in 6 cases combined with CD34 expression. Thus, in total, 101/112 patients were monitored for LSC frequency. After first cycle of chemotherapy, using a cut-off 5 × 10−6, patients with LSC frequency above cut-off (LSC+) showed shorter overall survival (OS, p=0.06) and especially relapse-free survival (RFS, p=0.001) as compared to LSC- patients. Median RFS was 11 months vs >36 months, respectively (fig A). Also after second induction cycle, high LSC frequency (>5 × 10−6) predicted shorter OS and RFS (both p<0.001). Median RFS of LSC+ patients was 8 months, that of LSC- patients >36 months (fig B). After consolidation therapy, 2 × 10−6 was the most optimal cut-off to define LSC+ and LSC- patients. OS and RFS were significantly shorter in LSC+ patients (p=0.037 and p=0.001, respectively), with a median RFS of 13 months vs. > 36 months (figure C). The relative risk of relapse was 5.0 (95% C.I 1.8–14.0) after first induction cycle, 4.7 (95% C.I.2.2-10.1) after second cycle and 8.5 (95% C.I 1.8– 41.4) after consolidation therapy. Multivariate analysis including WBC count, cytogenetic risk profile and the number of cycles needed to reach CR showed that LSC frequency is an independent factor for RFS (1st cycle p=0.01, 2nd cycle p<0.001, consolidation p=0.038). With this gating strategy, 90% of CD34+ AML patients could be monitored for residual LSCs, resulting in accurate prognostic information which is valuable for risk stratification-based treatment. In addition, the prospective isolation of LSCs and HSCs from diagnosis AML BM might identify more selective therapies that eradicate LSCs, while leaving HSCs intact.
This work was supported by Netherlands Cancer Foundation KWF.
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal