Abstract
Abstract 724
Heparin-induced thrombocytopenia and thrombosis (HITT) is a serious complication of heparin therapy, placing patients at increased risk for thrombosis and death. The chemokine platelet factor 4 (PF4) is central to the pathogenesis of HITT. Tetrameric PF4 forms ultralarge complexes (ULC) with heparin, which, when bound to specific antibodies, lead to thrombocytopenia and thrombosis. We have previously shown that the tetrameric form of PF4 is required for ULC formation. Since a large number of hospitalized patients are exposed to heparin, HITT is a major iatrogenic cause of morbidity and mortality in this patient population. There are currently no specific therapies for the treatment of HITT.
Identify small molecule antagonists of PF4 tetramerization to serve as reagents for the mechanistic study of HITT as well as lead compounds for development of novel HITT therapies.
Using the crystal structure of PF4, we identified a potential antagonist binding site near residues glutamic acid 28 (E28) and lysine 50 (K50). These residues form salt bridges between two PF4 dimers which are required for tetramer formation. We then performed an in silico screen of 1.1×10^6 lead-like small molecules using the program DOCK to identify compounds likely to bind to this site. A subset of these compounds were obtained and tested for their ability to inhibit PF4 tetramerization and PF4:heparin ULC formation in vitro.
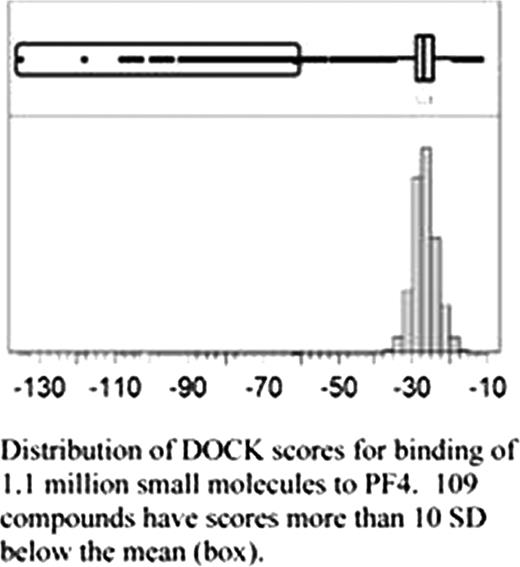
In silico screening identified 109 compounds with DOCK scores (lower scores predict higher binding affinity) which were greater than 10 standard deviations below the mean (see Figure). We have obtained 12 of these compounds for in vitro testing. The ability of antagonists to inhibit PF4 tetramerization was measured as previously described by quantitation of silver stained SDS-PAGE gels of cross-linked PF4 in the absence and presence of putative antagonists. 5 of the compounds showed inhibition of tetramerization in a dose dependent manner, with 2 of these compounds (PA31 and PA35) demonstrating micromolar potency (IC50 of tetramerization = 500uM and 600uM respectively). The ability of these antagonists to inhibit ULC formation between PF4 and heparin was measured by both gel filtration and ELISA as previously described. PA31 and PA35 both inhibited ULC formation with potencies similar to that for inhibition of tetramerization.
We have identified two small molecule PF4 antagonists. These compounds will be useful in future mechanistic studies of HITT. They also may serve as lead drug discovery compounds for chemical modification to create PF4 antagonists with increased affinity and potency. Such compounds may be tested as novel therapeutics for the treatment and/or prevention of HITT.
Rux:In sillico Molecular, LLC: Employment, Owner.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal