Abstract
Abstract 72
Studies of B-cell depletion using Rituximab in adults with ITP report responses lasting at least one year in almost all of the 30–40% of patients with complete responses (CR: platelet count >150 × 109/l) and also a small fraction of patients with partial responses (PR: platelet count 50–150 × 109/l). However data describing patients with ITP who are relapse-free and off-treatment beyond 1–2 years from initial Rituximab are almost entirely anecdotal and comparable response data are even less available for children. This study assessed the duration of unmaintained platelet response following rituximab treatment in 72 adults and 66 children with ITP, all of whom had had at least an initial response to rituximab. Long-term outcome was estimated from these data.
Seventeen published studies including 486 patients, 376 adults and 110 children, were used to obtain the initial response rates to standard-dose rituximab treatment (375mg/m2 weekly for 4 weeks) in adults and children. Only 1 included study did not use the standard dose of rituximab. The Godeau study (Blood, 2008) was used to estimate the one-year response rate in adults with ITP. Only those adults whose responses persisted at least one year had follow up assessed whereas children who demonstrated even ephemeral responses were included. Only verified counts were used in this IRB-approved multicenter study.
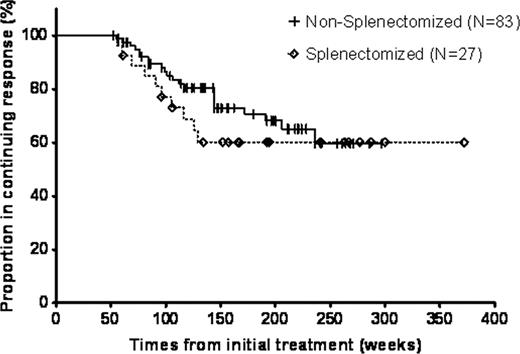
138 subjects with CR's or PR's after rituximab were included. All patients had starting platelet counts <30×109/l and 131 (95%) had ITP of > 6 months duration. Thirty-three (24%) had undergone splenectomy. Using the data from prior publications to obtain the initial response rates, children had a 56% initial response rate to rituximab treatment and adults had a 57% rate. Taking initial responders and then using the Godeau data for adults and Kaplan-Meier analysis of our data for children, 38% one-year response rates were obtained for both children and adults treated with rituximab. Both age groups also showed remarkable similarity at two years with 30% relapse-free response rates. However, all of the 26 eligible children maintained their response beyond two years whereas adults continued to relapse. Therefore the five-year response rate was 30% for children and only 21% for adults. Sex, duration of ITP, and age among adults did not affect long-term outcome. The rate of relapse was almost identical for splenectomized patients and non-splenectomized ones but the splenectomized patients appeared to relapse sooner (Figure). Patients with CR's (55 of the 72 adults with responses lasting at least one year were CR's) had better long-term outcomes than did patients with PR's even more than one year from initial treatment. B-cells returned significantly sooner to higher levels in subjects who relapsed compared to those whose responses were ongoing. No clinical long-term toxicity was observed but 2 patients were identified to have mild hypogammaglobulinemia > 30 months from initial treatment.
In summary, only approximately 1 in 5 adults treated with rituximab will have an at least five-year relapse-free response rate which is disappointingly low; children have only a slightly higher five-year relapse-free response rate. A pilot study to improve outcomes using either R-CVP or double dose rituximab was unsuccessful (Hasan, Am J Hematol,2009) Current efforts to improve long-term response rates have focused on the combination of high dose dexamethasone and rituximab (or even by providing maintenance treatment with rituximab). A better understanding of the mechanism of effect of rituximab in patients with ITP might allow an improved treatment strategy to be developed. Fortunately, the toxicity of rituximab treatment in patients with uncomplicated ITP appears to be low; however, yearly testing for immunoglobulins for a minimum of five years might be appropriate.
Kaplan-Meier Analysis of Long-term Response Duration Comparing Splenectomized to Non-splenectomized Patients after Initial Rituximab Treatment.
Kaplan-Meier Analysis of Long-term Response Duration Comparing Splenectomized to Non-splenectomized Patients after Initial Rituximab Treatment.
Neufeld:Novartis. Inc: Research Funding. Shenoy:Novartis Oncology: Honoraria. Bussel:Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal