Abstract
Abstract 7
Hispanic children with ALL have a significantly inferior outcome compared with non-Hispanic whites (Blood, 2002). Low systemic exposure to 6MP adversely affects prognosis (N Engl J Med 1990), and red cell (RBC) levels of its metabolite (thioguanine nucleotide [TGN]) correlate with survival (Blood, 1999). Significant inter-patient variability in RBC TGN levels exists due either to inherited difference in thiopurine methyltransferase (TPMT) activity, or failure to adhere to prescribed 6MP. Non-adherence to 6MP has been reported, and could be related to differences in cultural beliefs, possibly accounting for ethnic differences in ALL survival.
We tested this hypothesis in Hispanic and non-Hispanic white children with ALL diagnosed at age ≤21 yrs, and receiving maintenance therapy at 78 participating COG institutions. 6MP adherence was measured over 6 months using Medication Event Management System (MEMS) and RBC TGN. Electronic MEMS cap collected real-time data on dates/ times when the 6MP bottle was opened. Whether the bottle opening was followed by ingestion of 6MP was assessed by monthly (6 assessments/ patient) measurement of TGN. TPMT activity was also measured. Self-reported sociodemographics and reasons for non-adherence were also collected. MEMS-based adherence (outcome of interest) was defined as the ratio of days that 6MP bottle was opened to days 6MP doses were prescribed, reported as a percentage: “% adherence”. All prescribed doses were reviewed for each patient, and the period of time when 6MP was withheld by the prescriber due to toxicity/ illness taken into account. Longitudinal analysis was performed using Generalized Estimating Equations.
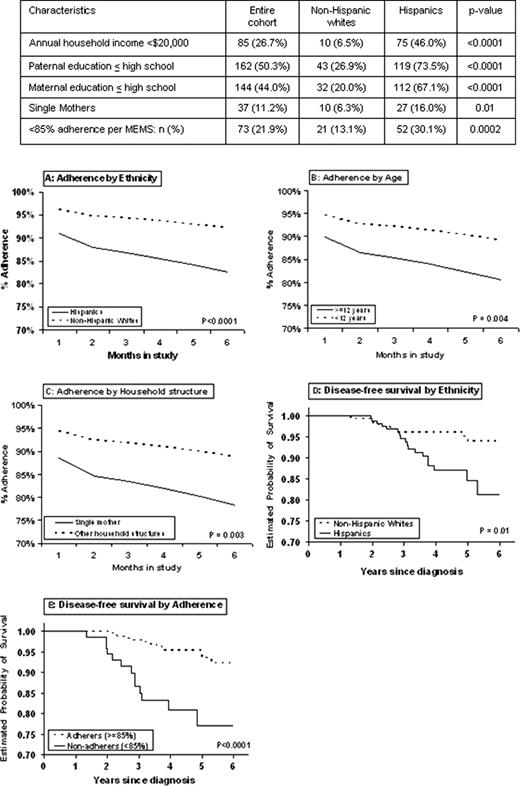
333 patients (173 Hispanics; 160 non-Hispanic whites) contributed 54,193 person-days of observation for 6MP adherence. Median age at study participation was 6 yrs (2-20); 67% were males; 38% presented with high-risk disease per NCI criteria. While age, sex and disease characteristics did not differ by ethnicity, Hispanics were significantly more likely to report indices of lower SES (Table). Mean % adherence over the 6 month study period was significantly lower among Hispanics (86±20%) compared with non-Hispanic whites (93±9%, p<0.0001). Multivariate longitudinal analysis identified the following at risk for lower adherence: Hispanic ethnicity (p<0.0001, Fig A); age ≥12 yrs at study entry (p=0.004, Fig B); single mothers as caregivers (p=0.003, Fig C); and time on study (p<0.0001). Reasons for missing 6MP doses included forgetfulness (55%), disruption of usual routine (22%), logistical barriers (15%), and side effects (5%). With a median follow-up of 4.5 yrs, disease-free survival (DFS) was significantly inferior for Hispanics (81±5% at 6 yrs) compared with non-Hispanic whites (94±2%, p=0.01, Fig D). After adjusting for sex, NCI risk, TPMT activity, and 6MP dose-intensity, each 1% decrease in adherence was associated with a 3% increase in risk of relapse (p=0.01). Most importantly, in the multivariate model without adherence, Hispanic ethnicity was associated with a significantly increased risk of relapse (HR=3.2, p=0.01); however upon inclusion of adherence in the model (p=0.01), the association between ethnicity and recurrence diminished in magnitude and significance (HR=2.1, p=0.18). A cutpoint in adherence at 85% was accompanied by a statistical separation in DFS (DFS: 92±2.2% vs. 77.0±5.1%, p<0.0001, Fig E), as well as a large separation in TGN levels (198 vs. 174, p=0.07), making 85% a clinically relevant level of adherence.
Non-adherence to 6MP is prevalent in children with ALL. Hispanic children are more likely to be non-adherent, even after adjusting for sociodemographic variables. Non-adherence is associated with significantly inferior DFS. Non-adherence explains the ethnic differences in DFS. This study has informed a targeted intervention to reduce non-adherence to oral chemotherapy, with the goal to reduce disparities in ALL outcome.
Relling:St. Jude Children's Research Hospital: Employment, Patents & Royalties; Enzon Pharmaceuticals: Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal