Abstract
Abstract 527
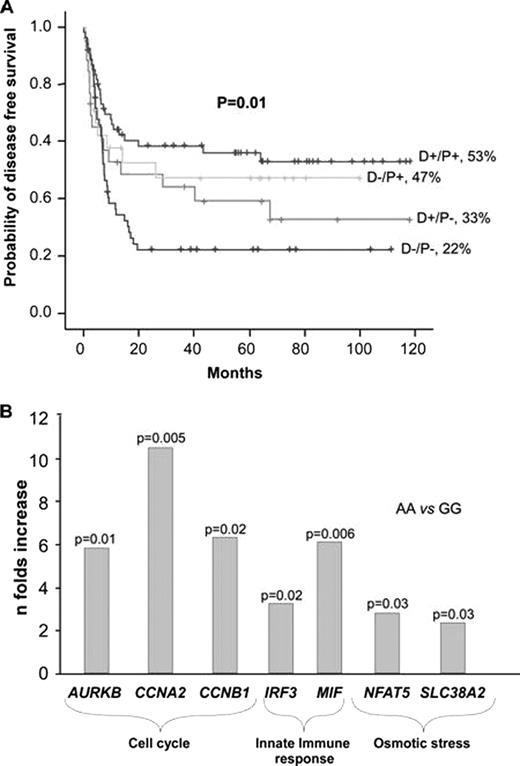
Despite considerable progress in the management of infections, in more stringent criteria for HLA compatibility, in the use of new immunosuppressive drugs and in tailored conditioning regimens, allo-SCT is still associated with a considerable morbidity and mortality, especially due to infection and leukemic relapse. Little is known about the relative importance of genetic characteristics that influence individual responses to infection and to malignant cells. We hypothesized that individual non-HLA genetic characteristics of the donor and/or recipient may influence the degree of inflammatory and antileukemic responses after allo-SCT. The objective of this study was to examine – in both the donor and the recipient – single nucleotide polymorphisms (SNPs) in genes involved in innate immunity (HAMP, IRF-3, PTX3, HBD1, TGFB1) and in transcriptional factors (TF) (ATBF1 and EP300) and to evaluate their influence on clinical outcomes after allo-SCT, specifically on the incidence of disease free survival (DFS), overall survival (OS), transplant-related mortality (TRM), and relapse. The study was performed in a first cohort of 106 donor-patient pairs (Hospital Virgen del Rocío, Seville) and was later validated in a second cohort of 99 donor-patient pairs (Hospital Clinic, Barcelona). In the first cohort, patient median age was 38 years (range, 5–66); 52% were in advanced phase of disease; and 29% received a reduced intensity conditioning regimen. Although several SNPs were associated with clinical outcome in this cohort, the strongest association was found with the transcriptional factor EP300. The dominant variant AA in rs7193297 in EP300 codes for a missense change. Patients with this variant attained a higher DFS (44% vs 37%, p=0.01; OR=2, p=0.03) and OS (54% vs 25%, p=0.009; OR=2, p=0.03) and showed a trend towards a lower TRM (16% vs 31%, p=0.1; OR=2, p=0.1). In the second cohort, median age was 45 years (range, 17–64); 55% were in advanced phase of disease; and 23% received a reduced intensity conditioning regimen. Results in this group of patients were very similar to those found in the first cohort. Patients with the dominant variant in EP300 attained a higher DFS (53% vs 24%, p=0.018; OR=2, p=0.03) and OS (65% vs 34%, p=0.016; OR=2, p=0.03) and showed a trend towards a lower TRM (21 vs 32%, p=0.3). When both cohorts were analyzed together, differences between patients with the dominant variant and those with the recessive variant were more significant. Those with the dominant variant had a higher DFS (53% vs 24%, p<0.0001; OR=2, p=0.004) and OS (54% vs 34%, p=0.001; OR=2, p=0.005) and showed a trend towards a lower TRM (18% vs 31%, p=0.07; OR=1.7, p=0.09) and a lower relapse rate (31% vs 45%, p=0.04; OR=1.6, p=0.05). When the analysis was performed according to the presence of the SNP in donor and/or in patient, the association of EP300 with clinical outcome was stronger when the variant was present in both donor and recipient (figureA). EP300 is a histone acetiltranspherase that regulates transcription via chromatin remodelling; it is involved in viral infection by interacting with IRF3 to activate interferon transcription and plays an important role in hemopoietic stem cell proliferation and differentiation. Due to the importance of EP300 in the regulation of mRNA expression of innate immunity and cell proliferation genes, we analyzed rs7193297 in EP300 by real-time RT-PCR in healthy individuals. The dominant variant was associated with higher expression of innate immune genes (IRF-3, p=0.02; MIF, p=0.006), cell cycle genes (AURKB, p=0.01; CCNA2, p=0.005; CCNB1, p=0.02) and osmotic stress genes (NFAT5, p=0.03; SLC38A2, p=0.03) (figureB). Our findings indicate a beneficial effect for the dominant variant in EP300 on clinical outcome after allo-SCT, which might be explained by its influence in the mRNA expression of innate immunity and cell proliferation genes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal