Abstract
Abstract 5176
The effect of complement on the immune system is complex and includes inhibition of aspects of the immune response. For example, we previously reported that C3b can inhibit the ability of mAb to activate NK cell-mediated antibody-dependent cellular cytotoxicity and that polymorphisms in C1q associated with lower C1q levels correlate with better therapeutic outcome. Polymorphisms in C5 and C9 complement components have also been shown to be associated with lymphoma risk. The current studies were designed to assess whether complement components may exert their inhibitory effects through the immune regulatory network, such as that mediated by T regulatory (Treg) cells.
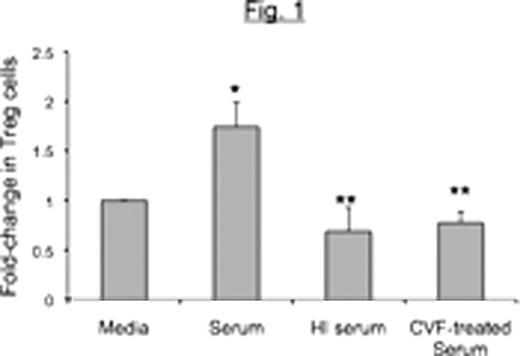
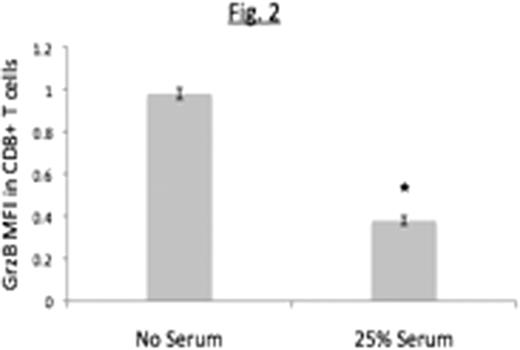
Peripheral Blood Mononuclear Cells (PBMCs) from healthy donors were co-cultured with Raji B cell lymphoma cells for 48 hours in the presence of unmodified autologous human serum or complement-depleted serum [heat-inactivated (HI) or cobra-venom factor (CVF)-treated serum]. Cells were stained with anti-CD3-APC-Cy7, CD4-PE-Cy7, CD25-PerCP-Cy5.5 and Foxp3-APC and the percent of CD4+ T-cells that were CD25highFoxp3+ (henceforth referred to as Tregs) determined. The ability of cells cultured in this manner to inhibit granzyme B expression in PHA-activated CD8+ autologous T cells was also determined.
unmodified or complement-inactivated human
We conclude that complement enhances generation of Treg cells induced by B lymphoma cells as indicated by both phenotypic and functional studies. Ongoing studies are exploring the role of individual complement components in Treg induction, and the possibility of using complement depletion as a strategy to boost immune responses against lymphoma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal