Abstract
Abstract 5025
Serum protein electrophoresis (SPE) is widely used for the detection and surveillance of patients with plasma cell dyscrasias. This study was initiated following the detection of a new IgG heavy-chain immunoglobulin in a 51-year old female patient with a known history of IgDk multiple myeloma. The laboratory finding was unusual because of the rarity of an apparent IgG heavy-chain gammopathy in combination with an IgDk as well as a low quantitative serum IgG of 255 mg/dL (reference interval 600–1700). Interference was suspected and after contacting the treating physician it was established that the patient was enrolled in a phase II clinical trial with the monoclonal antibody Situximab (Centocor, Inc., Malvern, PA, USA) in combination with dexamethasone. Siltuximab is a high affinity antibody to human interleukin-6 (IL-6) currently used in clinical trials for the treatment of a number of malignancies, including multiple myeloma. The objectives of this study were to determine if monoclonal immunoglobulin therapies interfere with SPE testing and commonly used immunoassays.
Siltuximab was obtained from the manufacturer and tested as an interferent using two different SPE platforms: Capillarys II (Sebia, USA) and Hydrasys (Sebia, USA). Siltuximab was analyzed alone or spiked into normal reference sera at concentrations between 50–600 mcg/mL to reflect the reported range of therapeutic circulating drug concentrations. We also examined additional monoclonal therapies at their reported mean peak serum drug concentrations. The therapies tested represented both chimeric human-mouse immunoglobulins (Rituximab, Siltuximab, Infliximab, Cetuximab) and humanized antibodies (Trastuzumab, Bevacizumab, Adalimumab). We also tested the effect of Situximab on a variety of automated serum immunoassays including hCG, iPTH, troponin T (Roche Diagnostics), cortisol, Hs-CRP, PSA, RF, and TSH (Ortho Clinical Diagnostics).
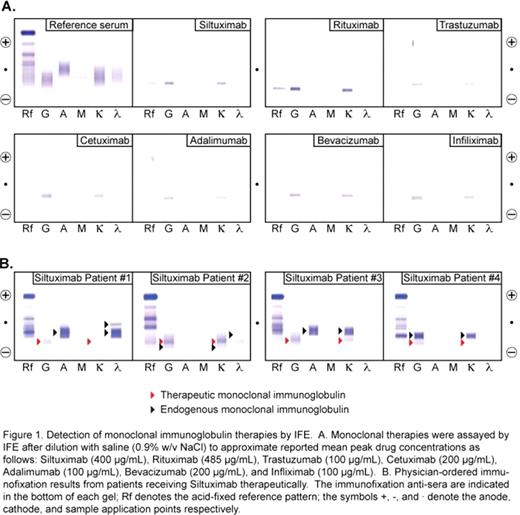
Siltuximab was evident as an IgGk monoclonal protein at a threshold of 100 mcg/mL and detected by all methods tested including capillary electrophoresis, immunosubtraction, agarose gel electrophoresis, and immunofixation (IFE). We also tested other widely prescribed monoclonal therapies at their reported mean peak serum drug concentrations: Rituximab (Rituxan), Trastuzumab (Herceptin), Bevacizumab (Avastin), Infliximab (Remicade), Cetuximab (Erbitux), and Adalimumab (Humira). Each drug was readily detected using IFE as an IgGk monoclonal protein (Fig 1A). Review of immunofixation results from 13 additional patients receiving Siltuximab revealed a discrete IgGk monoclonal protein corresponding to the migration of the drug in 11/13 patients (4 examples are shown in Fig 1B). In the other two cases, endogenous IgGk monoclonal immunoglobulins overlapped with the electrophoretic patterns of Siltuximab making them indistinguishable. We therefore tested two different approaches to cope with interference from monoclonal therapies: 1) adsorption of drug with specific antisera and 2) patient testing after allowing time for drug clearance. Pre-incubation of Siltuximab with anti-drug antibodies shifted the drug electrophoretic pattern such that it could potentially be differentiated from endogenous monoclonal proteins. We also observed that Siltuximab was undetectable by IFE in patients 3–4 months after cessation of therapy. This time period corresponds to approximately 5 half-lives (median half-life of siltuximab is 17.8 days). We observed no effect (<5% bias) from monoclonal therapies in commonly used immunoassays (hCG, PTH, troponin T, cortisol, Hs-CRP, PSA, RF, TSH) or quantitative immunoglobulin tests (IgG, IgA, IgM).
Interference of monoclonal antibody therapies may be of clinical significance in patients being investigated or monitored for plasma cell dyscrasias. Individuals receiving these therapies may be subjected to unnecessary follow-up testing and patients with an IgGk gammopathy may be misconstrued as having recurrent or resistant disease. This study highlights that the possibility of false positive testing on IFE needs to be considered given the increasing use of these agents in clinical practice. Pre-incubation with anti-drug antibodies or testing after clearance of the therapeutic immunoglobulins can help clarify concerns of monoclonal therapy interference with IFE testing.
Voorhees:Millennium Pharmaceuticals: Speakers Bureau; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal