Abstract
Abstract 4968
In our previous paper (Ferrero et al, BJH 2009) we reported the treatment of 63 MDS patients (median age 75, 16 RAEB1, 47 non RAEB) with a combination of human recombinant erythropoietin (alfa or beta epoetin, 30–80000 U/ week, median 65000U/week), 13-cis-retinoic acid (20 mg day) and dihydroxylated vitamin D3 (1 ug day). Eleven of the 16 RAEB1 patients also received intermittent, low dose of 6-thioguanine.
In spite of adverse prognostic factors for response to erythropoietin (all patients with Hb <9.5 g/dl, 70% transfusion dependent, 51% IPSS intermediate 1 or 2) 64% of non RAEB and 50% of RAEB1 displayed an erythroid response according to Cheson et al (Blood 2006).
At previous evaluation (41 months of follow-up) a survival advantage was evident for non RAEB patients with erythroid response. Now we updated the casistic after 3 years from the previous evaluation.
Median follow up for alive patients is now 64 months (5 months - 12 years). Median duration of erythroid response is now increased to 25 (2-88+) months for non RAEB and 6 (2.5-34.5+) months for RAEB1, 32.5% of responses in non RAEB patients have lasted more than 3 years.
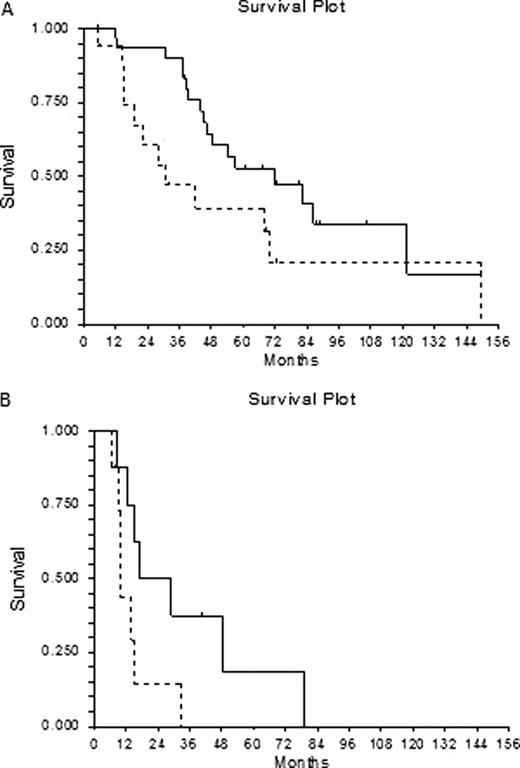
Twenty-nine/46 non RAEB and 14/16 RAEB1 patients died, with a median survival respectively of 57 and 15 months. Acute myeloid leukemia evolution occurred to 10 patients (5 RAEB1 and 5 non RAEB patients). Although the erythroid response did not correlate with known risk factors such as IPSS score, caryotype and transfusion requirement, it confirmed its positive prognostic role for survival in non RAEB patients (p 0.04, HR 2.06): median survival 71.5 months (range 12–150+) for responders, 30.6 months (range 5–149) for non responders.
A trend towards a better survival for responder was also observed among RAEB1 patients (median survival 17 months for responders, 10 months for non responders), however, due to the low numbers of patients in this group, the difference was not statistically significant, even if border line (p 0.052, HR 2.52).
Overall survival of myelodisplastyc patients according to erythroid response: A. Non-RAEB patients:“___” responsive patients, “—” not responsive patients (p 0.04, HR 2.06) B. RAEB patients: “___” responsive patients, “—” not responsive patients (p 0.05, HR 2.52)
Overall survival of myelodisplastyc patients according to erythroid response: A. Non-RAEB patients:“___” responsive patients, “—” not responsive patients (p 0.04, HR 2.06) B. RAEB patients: “___” responsive patients, “—” not responsive patients (p 0.05, HR 2.52)
Off Label Use: The use of 13-cis retinoic acid and 1; 25(OH)2 vitamin D3 in myelodisplastyc syndrome is off-label. In our study we used that drugs in combination with erythropoietin as differentiative agents.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal