Abstract
Abstract 4889
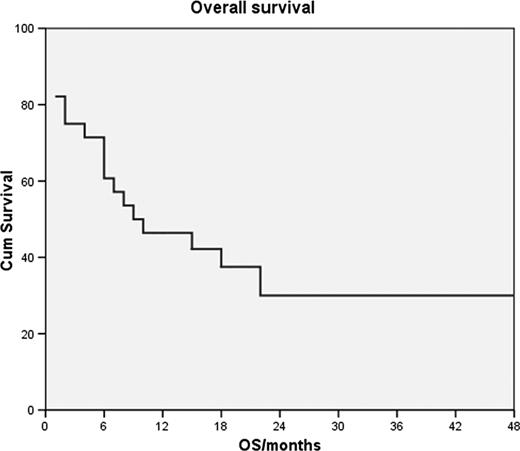
PTCLs, except ALK+ ALCL, have a bad prognosis with overall 5-year survival rates below 30%. Because of their relative rarity, no large randomized trials have been performed and the importance of individual cytotoxic agents, their dose density and intensity as well as that of autografting remains unproven. At our center we have been using dose-intense BACOP, a regimen modeled after the French ACVBP, for treatment of patients with high risk PTCL. The regimen consisted of cyclophosphamide 1500 mg/m2 day 1, doxorubicin 75 mg/m2 day 1, bleomycin 15 mg days 1 and 5, vincristine 2 mg days 1 and 5 and prednisone 50 mg/m2 days1-5 every 3 weeks for 4–6 cycles. Responding patients underwent stem-cell collection and autografting after BEAM conditioning. Areas of initially bulky disease and those not in CR prior to transplantation were irradiated posttransplant with 30–40 Gy. Patients with high risk for development of CNS disease received intrathecal methotrexate prophylaxis. Response evaluations were performed after 2–3 cycles and at the end of chemotherapy. Patients were considered high risk if they had any PTCL type with IPI 3 or more, bulky disease or failed to respond to 2–3 cycles of standard CHOP-21. We treated 28 patients, 20 men and 8 women, 17–67 years old (median 46). Thirteen had PTCL-NOS, 8 ALCL, 3 AILD, 2 nasal type, 1 panniculitis-like and 1 hepatosplenic PTCL. Two had stage IE bulky, 1 stage II bulky, 2 stage III and 23 stage IV disease. The toxicity of treatment was substantial, all patients experienced serious hematological toxicity and infections or neutropenic fever, 3 died of infection during chemotherapy and 1 during stem-cell transplantation, 2 had DVT/PE, 1 died of complications related to the necrosis of an extremely bulky tumor and one underwent emergency splenectomy after tumor necrosis caused splenic rupture. Three patients were unavailable for efficacy analysis due to early death, 4 progressed and 21 initially responded (75%); 14 achieved CR and 7 PR. None of the 3 patients with nasal type or hepatosplenic PTCL responded. Of the responders, 11 underwent autografting as intended, 3 relapsed prior to transplantation, treatment was modified in 3 due to toxicity, 2 were not transplanted due to patient refusal or physician decision, 1 underwent allogeneic transplantation and 1 failed to mobilize. After a median follow-up of survivors of 20 months, 9 patients are still alive, including 8 who were autografted as intended. Median response duration was 12 months, FFS 6 months and OS 9 months (Fig). In conclusion, a significant proportion of high-risk PTCL-NOS, ALCL and AILD patients respond to dose-intense BACOP followed by autologous stem-cell transplantation but the toxicity of treatment is substantial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal