Abstract
Abstract 4560
About 15% of acute myelogenous leukemia (AML) patients will have disruption of the core binding factor (CBF) transcription factor as indicated by the detection of t(8;21) or inv(16) on metaphase cytogenetic analysis. In general, patients with CBF-AML are younger and regarded as having a favorable outcome when treated with anthracycline-based induction chemotherapy and multiple cycles of high-dose cytarabine consolidation. Autologous and allogeneic hematopoietic stem cell transplantation (HSCT) are usually perceived as unnecessary in the management of CBF-AMLs. However, most data indicating the favorable prognosis of CBF-AML with non-HSCT management originates from young selected patients participating in clinical trials and information on relapse treatments is often lacking. We performed a retrospective analysis of consecutive patients diagnosed with CBF-AML at a regional leukemia center over the last twelve years to assess long term survival of unselected patients and the likelihood of therapeutic success without ever requiring HSCT.
The study cohort was identified through a search of all AML reports issued by the institutional clinical cytogenetics laboratory since the introduction of electronic records (1998-2010). We subsequently reviewed the charts of all patients with CBF-AML (irrespective of other cytogenetic abnormalities) to extract demographic, treatment, and outcome information. Survival status and subsequent treatments in other institutions were determined by contacting the patient, attending physician or review of public death records. Data collection and analysis were approved by the Institutional Review Board.
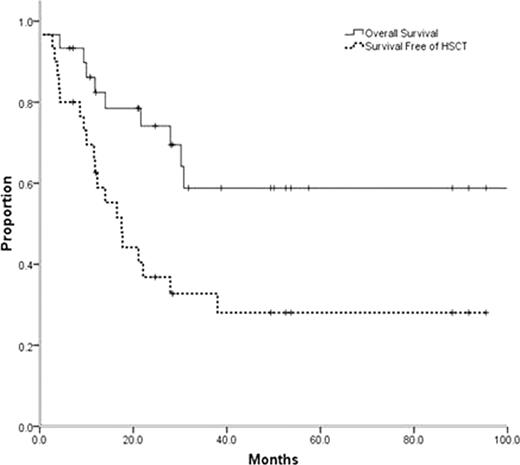
Thirty patients with CBF-AML were identified, 14 with t(8;21) and 16 with inv(16) AML. Median follow up for survivors in this series is 35.3 months (range 6.4–117.4) with all survivors currently in remission. Median age at diagnosis was 43 years (range 18–69) with 10 (33%) patients older than 55. All patients initially received therapy with curative intent with anthracycline-based induction chemotherapy. Only 47% of the patients were treated on clinical trials. There was 1 death during induction therapy and 22/29 patients (73.3%) achieved a complete remission with initial induction therapy. Overall 13 patients (43.3%) have required some modality of HSCT. Six patients received HSCT in CR1 due to perceived high risk of relapse (5 autologous, 1 allogeneic). None of the 6 patients who underwent HSCT in CR1 (5 autologous, 1 allogeneic) have relapsed. Among the 16 patients receiving non-transplant consolidation in CR1, 6 have subsequently relapsed and required a HSCT (3 autologous, 3 allogeneic) and 3 of these patients are alive 20.9–117.4 months from the initial diagnosis. Seven patients received HSCT in CR2 or in relapse (4 autologous, 3 allogeneic) and 6 of these patients are long-term survivors. Estimated 5 year overall survival for the entire cohort is 58.8 +/− 10.8%, comparable to what has been described for younger patients entering clinical trials (Grimwade et al, 2010). However, the likelihood of survival at 5 years without requiring HSCT was only 28.1+/− 8.8% (Figure).
The favorable outcome previously reported for CBF-AMLs was reproducible in unselected patients. However, only a minority of patients were long term survivors relying exclusively on conventional chemotherapy. In the near future, strategies for molecular risk stratification of CBF-AML patients need to be coupled with risk-adapted therapy, likely including early use of HSCT for high-risk patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal