Abstract
Abstract 4457
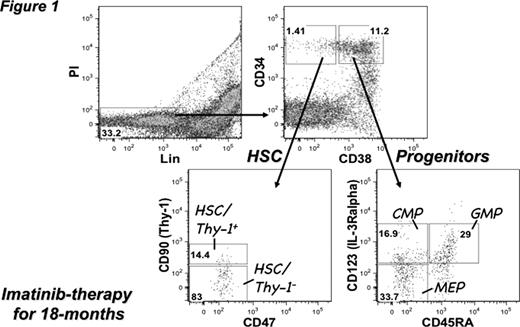
Chronic myeloid leukemia (CML) is effectively treated with imatinib (IM), however, several mathematical models and ex vivo-examinations suggested that IM-therapy does not eradicate BCR-ABL-positive hematopoietic stem cells (HSC). We prospectively (0, 3, 6 and 12 months after IM-therapy) investigated 16 newly diagnosed and 22 long-term followed CML-chronic phase (CP) cases using methods previously reported (Jamieson et al., N Engl J Med, 2004. and Abe et al., Int J Hematol, 2008) (Figure 1) with FACSAria™ and quantitative RT-PCR of BCR-ABL among each sorted population; total mononuclear cells, HSC/Thy-1+, HSC/Thy-1–, common myeloid progenitors (CMP), granulocyte macrophage progenitors (GMP) and megakaryocyte erythroid progenitors (MEP). In optimal responders to IM-therapy, BCR-ABL transcripts in the HSC populations (HSC/Thy-1+ and HSC/Thy-1–) tended to be more retentive than other populations while gradual reduction was observed during the first 12 months in all populations. And discrepancy of minimum residual diseases (MRD) between the HSC populations and other populations was larger in patients after longer IM-therapy. In evaluating properties of CML stem cells and other markers, we observed irrelevant distribution of side population (SP) and expressions of ABC transporters (ABCB1 and ABCG2) in comparison with CD34/38 expression. We also prospectively investigated BCR-ABL transcripts in each population of 23 IM-resistant or -intolerant CML-CP cases and one newly diagnosed CML-accelerated phase (AP) case during treatment with second-generation tyrosine kinase inhibitors (2nd TKIs), dasatinib or nilotinib. Treatment with each inhibitor induced more rapid reduction of BCR-ABL transcripts even in the HSC population (CD34+CD38–) during the first 6 months and there was no significant difference of MRD among each population in optimal responders to 2nd TKIs-therapy. In the stromal co-culturing system using primary cells and leukemic NOD/SCID/IL2rgnull (NOG) mice xenotransplanted with Ph+ leukemia cells, retention of quiescent slow-cycling (Hoechst 33342low/Pyronin Ylow) CD34+ population after IM-treatment were observed and cell death mechanisms after treatment with 2nd TKIs are also under investigation. These results imply that therapy with 2nd TKIs could be a promising approach for quick and efficient reduction of the CML stem cells and cure of disease.
Naoe:Kyowa-Kirin: Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal