Abstract
Abstract 443
AZA is the current standard of care for IPSS int-2 and high (“higher”) risk MDS. However, most pts will experience primary or secondary treatment failure. To date, there is no published data on the outcome of those pts.
565 patients from 4 independent cohorts, who had not responded to or relapsed after response to AZA, were included. The datasets included 3 prospective trials (AZA001 (n=138; Lancet Onc, 2009), J9950 (n=26; Cancer Res 2006), J0443 (n=32; NCT00101179) trials) and data from the French ATU compassionate use program (n=369). Two cohorts administered AZA as single agent (AZA001 and French ATU) while two combined AZA with a histone deacetylase inhibitor (J9950, sodium phenylbutyrate; J0443, entinostat). 339 patients had no clinical response to AZA while 226 relapsed after initial response. The influence of pre treatment variables and salvage treatment options on the outcome after AZA failure was analyzed. Survival was measured from the date of failure of AZA.
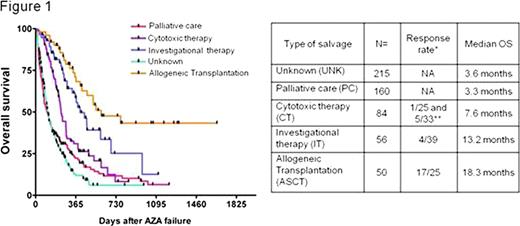
The cohort included 475 MDS (including 117 RAEB-T) and 90 sAML (AML secondary to MDS). Median age was 69 years and the median number of cycles of AZA before failure was 6 (range [1-41]). Patients were randomly assigned to one learning (n=377) and one validation (n=188) set stratified on the number of deaths. There was no difference in the baseline characteristics of the 2 sets. With a median follow-up of 15 months after AZA failure, the median overall survival (OS) was 6 months and the 2-year probability of OS was 15%. The multivariate model constructed with the learning set showed that age (HR 1.02/y, 95%CI [1.01-1.03], p=0.002), sex (female 7 months vs male 5.6 months HR 1.3 [1.01-1.67] p=0.04), cytogenetics (favorable 8 months vs intermediate 5.9 months HR 1.49 [1.06-2.08] p=0.02, vs high risk 4.6 months HR 2.19 [1.63-2.91] p<0.001), bone marrow blast % before AZA (below 10% 7.8 months vs 10 to 20% 5.8 months HR 1.34 [0.97-1.84] p=0.07, vs 21%+ 4.3 months HR 1.92 [1.36-2.7] p<0.001), and the number of cycles of AZA (6 or less 4.7 months vs more 7.2 months HR 0.69 [0.54-0.9] p=0.005) were significantly associated with survival. Initial response to AZA was significant in univariate analysis (non responders 4.7 m vs 7.3 m in responders, p=0.02) but did not retain significance in the multivariate model. These results were confirmed with the validation set. Data on the treatment administered after AZA failure were available for 350 patients. Allogeneic transplantation (n= 50) (median OS 18.3 months, range [3-55+]) and investigational therapies (n= 56, including epigenetic drugs, IMIDs, and non registered compounds; median OS 13.2 months, range [1-36+]) were associated with significantly better survival than palliative care (median OS 3.3 months) or conventional cytotoxic chemotherapy (median OS 7.6 months, including AML like induction chemo and low dose chemo such as cytarabine or 6-MP).
Outcome of patients with AZA failure was poor, although pts receiving allo SCT or investigational treatments had a somewhat better outcome. This work highlights the potential predictors of outcome and defined the baseline survival data that will help in the design of second line trials in higher risk MDS having failed treatment with AZA.
Survival analysis according to the salvage treatment regimens.
Survival analysis according to the salvage treatment regimens.
*: Overall response rate for each treatment group is presented with the number of patients evaluable for response in each cohort. For the CT group, response rate for low dose chemotherapy and high dose (AML like, marked with **) chemo have been individualized. Univariate analysis (log-rank test) showed significant difference between PC and CT (p=0.002), IT (p<0.001) or ASCT (p<0.001). There was also significant differences between CT and IT (p=0.004) or ASCT(p=0.001). Difference between IT and ASCT reached borderline significance (p=0.053).
Gore:Celgene: Research Funding, Stock options. Beach:Celgene: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal