Abstract
Abstract 425
Over 400,000 people in the United States are living with non-Hodgkin's lymphoma (NHL). While survival rates have improved, over 20,000 persons die annually from NHL. Cytotoxic chemotherapies are initially effective, but resistance can develop and dose limiting toxicities are problematic. Monoclonal antibody (mAb) therapy with rituximab, a chimeric anti-CD20 mAb, has shown benefit alone and in combination with chemotherapy, and can improve overall survival. New mAbs are being tested in combination with rituximab, including bispecific antibodies (BsAb) that simultaneously target CD20 and CD22. CD22 is a B-lymphocyte specific adhesion molecule expressed on nearly all mature B-cells, including the majority of B cell-NHLs. HB22.7 is an anti-CD22 mAb that specifically blocks the interaction of CD22 with its ligand, initiates CD22-mediated signal transduction, and has direct cytotoxic effects. Anti-CD22 mAbs that do not block ligand binding possess only modest functional effects; prior BsAbs contained non-ligand binding anti-CD22 mAbs. Therefore, we constructed a BsAb (Bs20x22) using rituximab and HB22.7, and evaluated its cell binding, signaling patterns, and lymphomacidal activity using a human NHL xenograft model.
Bs20x22 was constructed from F(ab')2 fragments of rituximab and HB22.7 using the ImmunoPure F(ab')2 Preparation Kit (Pierce). Cell binding studies used the CD20/CD22 double positive human Burkitt's B-cell lymphoma lines Raji and Ramos, and the CD20/CD22 double negative human embryonic kidney cell line, 293T. In vitro cytotoxicity was assessed by trypan blue exclusion. In vitro apoptosis assays were performed on plated Raji or Ramos cells which were then treated with HB22.7, rituximab, HB22.7 plus rituximab, or Bs20x22. The total number of cells and the number of apoptotic cells were counted, with results expressed as the % of control (% apoptotic cells treated / % apoptotic cells untreated).
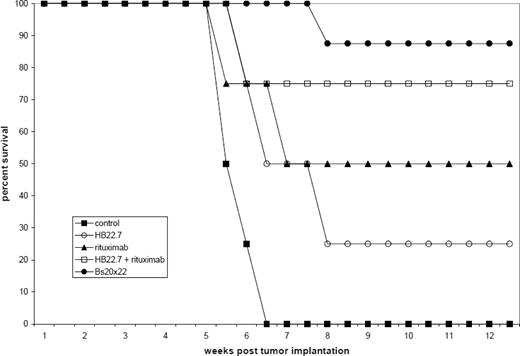
Bs20x22 specifically bound CD20 and CD22, similar to the parent mAbs rituximab and HB22.7. In vitro cytotoxicity assays showed that Bs20x22 was three times more effective than either parent mAb alone and twice as effective as a combination of both parent mAb used at equimolar concentrations. Additionally, Bs20x22 was nearly four times more effective at inducing apoptosis than either mAb alone, with the percentage of apoptotic cells greatest for Bs20x22 treatment (20.1%), compared to combination rituximab plus HB22.7 (7.5%), rituximab (6.7%), and HB22.7 (6.5%). Examination of the MAPK and SAPK signaling cascades revealed that treatment with Bs20x22 resulted in significant activation of p38, while treatment with either parent mAb did not. In an in vivo human NHL xenograft model, treatment with Bs20x22 resulted in significantly greater tumor shrinkage resulting in the smallest tumor volume of any group. Bs20x22 treated mice had the best survival rate (88%) compared to the combination of rituximab plus HB22.7 (75%), rituximab (50%), HB22.7 (25%), and PBS control (0%). Significantly greater efficacy was also found when Bs20x22 was administered prior to the development of visible tumors versus treatment of established tumors, with tumors 89% smaller in mice treated pre-emptively.
This study demonstrates the feasibility of constructing a bispecific antibody targeting both CD20 and CD22, using an anti-CD22 mAb with ligand-blocking ability. Efficacy was demonstrated by in vitro cytotoxicity and apoptosis assays, p38 activation, and human NHL xenograft models. Our results suggest that Bs20x22 is more efficacious than the combination of rituximab and HB22.7, and use of Bs20x22 eliminates the need for sequential administration of two separate mAbs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal