Abstract
Abstract 4249
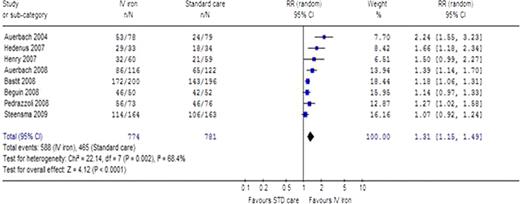
Anemia is an almost universal complication in cancer patients and an important contributor to morbidity of malignancy. For chemotherapy induced anemia (CIA), currently available guidelines recommend the use of erythropoiesis stimulating agents (ESAs), but are less certain regarding intravenous (IV) iron administration. Methods: Systematic review and meta-analysis of randomized controlled trials comparing IV iron with no iron or oral iron or for the treatment of CIA. A comprehensive search of The Cochrane Library, MEDLINE, conference proceedings and references was conducted until 2010. Two reviewers appraised the quality of trials and extracted data. Outcomes assessed were: number of patients achieving a hematopoietic response (defined as a hemoglobin level increase by more than 2gr/dl or an increase >12gr/dl), number of patients requiring blood transfusions, ferritin level, transferrin saturation, all-cause mortality, adverse events. For dichotomous data, relative risks (RR) with 95% confidence intervals (CIs) were estimated and pooled, for continuous data weighted mean differences (WMDs) were calculated. Results: Ten randomized trials including 1637 patients and conducted between the years 2004 and 2009 were included. The intervention was IV iron dextran in two trials, ferric gluconate in three trials, iron sucrose in four and one trial assessed both ferric gluconate and iron sucrose. ESAs were administered in nine trials. Most trials excluded patients with true iron deficiency. Treatment with IV iron significantly increased the number of patients achieving a hematopoietic response (RR 1.31 [95% CI 1.15, 1.49], 8 trials, figure), and significantly reduced the number of patients who required blood transfusions (RR 0.77 [95% CI 0.64, 0.94], 9 trials). When analyzed according to type of ESA administered to both arms of the trials, there was a consistent significant increase in the number of patients with a hematopoietic response in the IV iron arm, for all ESA types: darbepoetin alpha (RR 1.18 [95% CI 1.11, 1.26], 5 trials), epoetin alpha (RR 1.85 [95% CI 1.25, 2.74], 2 trials), epoetin beta (RR 1.66 [95% CI 1.18, 2.34], 1 trial). The ferritin level at the end of the trial was significantly increased in the IV iron arm compared with the control arm (WMD 234.47 [95% CI 136.94, 332.01], 4 trials), as was transferrin saturation(WMD 9.98 [95% CI 9.21, 10.75] 3 trials). There was no difference in all-cause mortality at the end of follow-up between the IV iron arm and the control arm (RR 1.01 [95% CI 0.65, 1.56], 6 trials). There was no difference in the rate of serious adverse events requiring intervention (RR 1.11 [95%CI 0.93, 1.31], 7 trials), in the occurrence of thromboembolic events (RR 1.03 [95%CI 0.59, 1.80], 4 trials) or cardiovascular events (RR 0.87 [95%CI 0.45, 1.70] [0.40-3.39], 5 trials). Conclusions: Our systematic review demonstrates better hematopoietic response and less need for transfusion, with no difference in mortality or adverse events. Thus, our results support the use of IV iron for the treatment of cancer patients with anemia.
Number of patients who achieved a hematopoietic response in the IV iron arm compared to control
Number of patients who achieved a hematopoietic response in the IV iron arm compared to control
Shpilberg:Roche: Consultancy, Honoraria.
This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal