Abstract
Because MDS are clinically heterogenous, classification and prognostic tools are essential to guide disease management decisions. The international, multicenter, phase 3 trial (AZA-001) enrolled 358 pts with higher-risk MDS as defined by French-American-British (FAB) criteria and International Prognostic Scoring System (IPSS) (Lancet Oncol 2009;10:223). The recent World Health Organization (WHO) classification of hematologic malignancies criteria (JCO 1999;17:3835) and the WHO Classification–Based Prognostic Scoring System (WPSS) (JCO 2007;25:3503) are increasingly being used in clinical practice.
To evaluate whether overall survival (OS), hematologic improvement (HI), and transfusion independence (TI) in the subgroup of patients (pts) from AZA-001 who met WHO classification and WPSS criteria for higher-risk MDS are consistent with overall findings from AZA-001.
According to protocol, AZA-001 enrolled pts with FAB-defined RAEB, RAEB-t, or CMML with more than 10% blasts, and IPSS Int-2 or High risk (Lancet Oncol 2009;10:223). Pts were randomized to AZA 75 mg/m2/d SC × 7d q 28d (n=179) or to a conventional care regimen (CCR; n=179), which included supportive care, low-dose ara-C, or intensive chemotherapy. This analysis included pts with WHO-defined RAEB-1 or RAEB-2 (pts with WHO-AML, CMML-1, CMML-2, or indeterminate classification were excluded) who had baseline WPSS risk of intermediate, high, or very high. Kaplan-Meier methods were used to estimate median OS and 95% CIs. HI and TI were defined by IWG-2000 criteria and results for AZA vs CCR were compared by Fisher's exact test. P values can only be used as a reference because these analyses were performed post hoc on a selected pt population that met WPSS criteria (40% of all AZA-001 pts did not meet these criteria and were excluded from analyses).
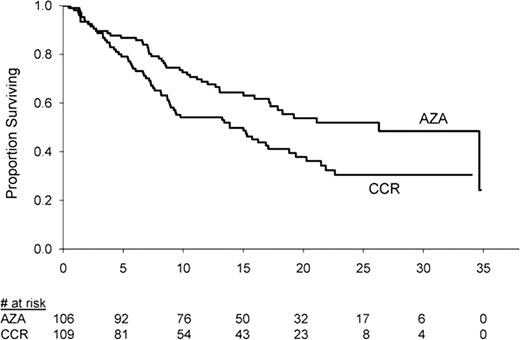
Overall, 215 pts in AZA-001 (106 AZA, 109 CCR) met a WHO classification for higher-risk MDS (RAEB-1: 13 AZA, 16 CCR; RAEB-2: 93 AZA, 93 CCR). Baseline WPSS risk was intermediate (1 AZA pt), high (66 AZA, 59 CCR), or very high (39 AZA, 50 CCR), with proportionately more CCR pts at very high risk than AZA pts (46% vs 37%, respectively). Median age was 68 years (range 42 – 83) in the AZA group and 70 years (range 38 – 88) in the CCR group. At baseline, 63% of both the AZA and CCR cohorts were RBC transfusion-dependent and 19% and 15%, respectively, were platelet transfusion dependent. Median OS was 26.3 months (95%CI: 17.2, not reached) with AZA vs 13.9 months (95%CI: 8.9, 18.8) with CCR, (log-rank p=0.016) (Figure ). Rates of any HI and major erythroid and platelet lineage responses were better with AZA vs CCR (Table). RBC TI was also more frequent with AZA than with CCR (Table), but the platelet TI rate did not differ between treatment groups.
These results are highly consistent with reported OS and hematological response outcomes for the entire AZA-001 population of higher-risk MDS pts classified by FAB and IPSS risk. The efficacy of AZA is consistently superior to CCR in pts with higher-risk MDS, irrespective of the classification and prognostic criteria used to describe them.
Gattermann:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sanz:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Giagounidis:Celgene: Consultancy, Honoraria. Seymour:Celgene: Honoraria, Speakers Bureau. Fenaux:Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Merck: Honoraria; Cephalon: Honoraria; Novartis: Honoraria; J&J: Honoraria. Santini:Celgene: Honoraria. Mufti:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Muus:Celgene: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Ramos:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Lucy:Celgene: Employment, Equity Ownership. Beach:Celgene: Employment. Silverman:Celgene: Honoraria.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal