Abstract
Abstract 3954
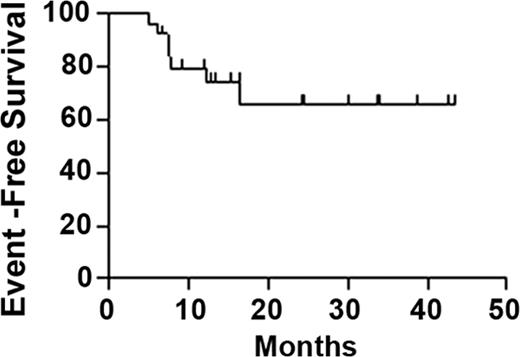
Background: Curative but less toxic regimens are needed for relapsed cHL, as autologous stem cell transplantation (auto SCT) carries ∼ 5% mortality risk and nearly 100% risk of infertility. Work at our institution showed that 1) high-dose Cy, equivalent to transplantation doses, can be given safely without SCT and the potential of reinfusing tumor cells, does not preclude any form of salvage SCT, and can spare fertility; and 2) unlike Hodgkin and Reed Sternberg (HRS) cells, cHL cancer stem cells may have a CD20+, memory B cell phenotype and circulate in surprisingly high frequencies (Jones RJ et al, Blood 2009;113:5920), potentially explaining rituximab's activity in this disease. Derived from the K562 cell line, the HL vaccine is a novel, allogeneic (allo), GM-CSF producing vaccine that expresses antigens commonly present in cHL including survivin. In addition, the vaccine expresses the EBV antigens LMP2 and EBNA1, commonly present in EBV+ cHL. Patients and Methods: A phase I/II study of high-dose therapy and immunotherapy for relapsed cHL was developed with curative intent. Eligibility included chemosensitive relapse (chemosensitivity testing was not required for low-volume relapse), no prior SCT, no primary refractory disease, and adequate end-organ function. Chemotherapy consisted of rituximab 375 mg/m2 IV on d 1 and 4, high-dose Cy (50 mg/kg/d IV on d 8–11) with mesna, filgrastim, and rituximab 375 mg/m2 IV weekly for 4 weeks upon platelet recovery. HL vaccine (1 × 10exp8 cells/dose) was administered 1 d after Cy completion and 4, 8, 12, 16, and 24 weeks later. Consolidative radiation was permitted. All treatment was intended to be outpatient. Results: Clinical outcomes of the first 25 pts accrued are described. Median age was 40 (range 18–67) y; 10 were female. Stage was IIB-IV in 14 pts (56%) upon diagnosis, and 9 pts (36%) relapsed with extranodal disease. HRS cells were CD20+ in 4/25 cases (16%), EBV+ in 6/24 evaluable cases (25%). Median time between first-line therapy completion and relapse was 12 (range 2–94) mo; 22 pts had CR or PR and 2 had stable disease to salvage therapy consisting chiefly of ICE or ESHAP (median 2 cycles, range 1–4), and 1 had untested relapse. With median 31 (range 7–53) mo follow-up for all pts, actuarial 1 y and 2 y overall survival is 100%, while actuarial event-free survival is 79% at 1 y and 66% at 2 y following high-dose Cy (Figure). The regimen was well-tolerated, and there was no treatment-related mortality. Eleven admissions occurred, 10 for infections (8 neutropenic, 2 non-neutropenic) which all resolved without sequelae, with 2 other neutropenic fevers managed without admission. One pt developed idiopathic protracted pancytopenia (gradually improving), cardiomyopathy, and chronic kidney disease. Vaccine toxicities were largely limited to mild local skin reactions. Median time to neutrophil recovery after the last dose of Cy was 17 (range 10–26) d; median time to sustained platelets >= 20,000/μL, without transfusion in the preceeding week, was 21 (range 0–46) d, with 5 pts not requiring platelet transfusion. Grade 3 or 4, late-onset neutropenia, presumably rituximab-related, was documented in 11 pts (44%; grade 4 in 5 pts) at a median of 3 (range 1–18) mo following study initiation. In the 7 relapsed pts, median time to relapse was 7 (range 5–16) mo after high-dose Cy; 1 died of disease after auto and allo SCT, 1 died in the course of allo SCT, 1 is alive with disease after allo SCT, 3 are event-free 7 mo - 3 y after allo SCT, and 1 has allo SCT planned. Early stopping rules for safety and efficacy have not been met. One pt had a successful pregnancy 33 mo after high-dose Cy. Conclusion: A novel outpatient regimen incorporating high-dose Cy and rituximab produces encouraging outcomes on preliminary analysis in relapsed cHL pts. Early efficacy appears comparable to that of auto SCT, with potentially less toxicity. Analyses of immunologic endpoints and HL biomarkers are in progress.
Kasamon:Genentech: Research Funding. Off Label Use: Rituximab, cyclophosphamide: off-label use. Cancer vaccine: investigational. Levitsky:HL vaccine: Patents & Royalties. Borrello:HL vaccine: Patents & Royalties. Swinnen:Genentech: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal