Abstract
Abstract 3912
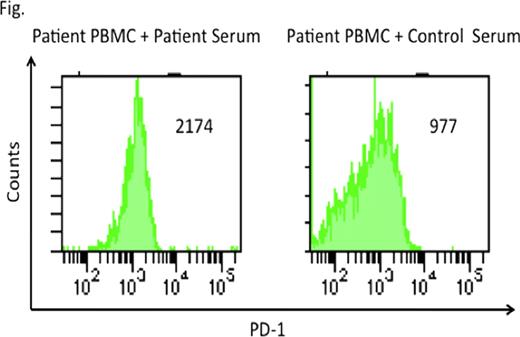
Cytomegalovirus (CMV) is one of the most common pathogens causing morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT), despite preemptive treatments employing antiviral drugs. Cytotoxic T cells are indispensable to control CMV infections. Chronic viral infections with human immunodeficiency virus or hepatitis C virus were shown to be associated with exhausted T cells with high expression of the inhibitory molecule programmed death 1 (PD-1). Recently, it has been reported that PD-1 up-regulation on CMV specific T cells was associated with CMV infection after renal and liver transplantation. PD-1 expression on CMV specific T cells after HSCT has not been well examined. We evaluated the involvements of exhausted CMV specific T cells characterized by high PD-1 expression in persistent CMV infection after allogeneic HSCT. Peripheral blood mononuclear cells (PBMC) and serum were obtained from an HLA-A*2402-positive patient who had received bone marrow transplantation from an HLA-A, B, C and DR matched unrelated donor. This patient failed to eliminate CMV for more than one year after transplantation despite intermittent administration of ganciclovir and foscarnet. Control PBMC and serum were obtained from an HLA-A*2402-positive healthy volunteer because the Japan Marrow Donor Program prohibits blood collection for research use from donor. All blood was collected with written informed consent. We at first analyzed frequencies of CMV-specific CD8+ T cells in patient and control PBMC by flow cytometer using QYDPVAALF/A*2402-specific tetramer and CD8 antibodies. QYDPVAALF is derived from CMV pp65 protein and presented by the HLA-A*2402 molecule. Tetramer stained cells were detected in the patient PBMC but control PBMC (0.11% versus undetectable). Patient and control PBMC were stimulated by a synthetic peptide QYDPVAALF in culture media containing IL-2 for 14 days, and stained with QYD/A*2402-specific tetramer. Remarkably, post-stimulated patient PBMC contained only 0.54% of tetramer stained CD8+ T cells, whereas a more dramatic increase (14.1%) in control PBMC. We analyzed frequencies of IFN-g secreting CD8+ T cells in PBMC after stimulation with a peptide pool covering the whole CMV pp65 protein for 4 hours. Less patient CD8+ T cells produced IFN-g, compared with the control CD8+ T cells (0.5% versus 1.1%) These data demonstrate dysfunction of CMV-specific CD8+ T cells in the patient with persistent CMV infection. To examine the mechanism of dysfunction of CMV-specific CD8+ T cells, we analyzed the expression of PD-1 on CMV-specific CD8+ T cells 14 days after stimulation with QYDPVAALF peptide. Multiparameter flow cytometry and tetramer assay exhibited higher expression of PD-1 on CMV-specific CD8+ T cells generated from patient PBMC, compared with CMV-specific CD8+ T cells generated from control PBMC. To find out whether the engagement of PD-1 to its ligand (PD-L1) leads to T cell exhaustion, we stimulated patient PBMC with QYDPVAALF peptide for 14 days in the presence or absence of anti PD-L1 antibody which blocks PD-1/PD-L1 inhibitory pathway. Blockade of PD-1/PD-L1 pathway resulted in 3.9-fold increase in patient CMV specific T cells. These findings demonstrate that PD-1 is associated with the exhaustion of CMV specific CD8+ T cells during persistent CMV infection in this patient. To examine the effect of patient serum on CMV specific CD8+ T cells, we stimulated patient PBMC with QYDPVAALF peptide for 14 days in culture media with patient or control serum. CMV specific CD8+ T cells increased 4-fold and 55-fold in the presence of patient and control serum, respectively. Patient serum led to higher PD-1 expression on CMV specific CD8+ T cells, compared with control serum (Fig). These findings suggest that patient serum may contain what regulates PD-1 expression level of exhausted T cells. Further investigations to identify factors regulating PD-1 expression in patient serum are in progress. The identification of the factors may provide new strategies to improve exhausted T cell function.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal