Abstract
Abstract 370
Light chain (AL) amyloidosis is characterized by the deposition of amyloid derived from immunoglobulin light chains in various organs. Autologous peripheral blood stem cell transplantation (SCT) is a commonly used and effective treatment for AL. For patients (pts) undergoing this procedure, a proportion of pts receive treatment similar to induction therapy employed in pts with myeloma undergoing SCT. However, the significance of induction therapy and its impact on the overall survival (OS) in AL is unknown. We conducted this study to ascertain the role of pre-SCT therapy on the post-transplant outcomes in AL.
Patients and methods: 435 pts with AL who underwent SCT at our institution between March 1996 and May 2010 form the study group. Groups were compared using Fisher's exact test or t-test, and survival was calculated using Kaplan Meier method. Survival curves were compared using log rank test.
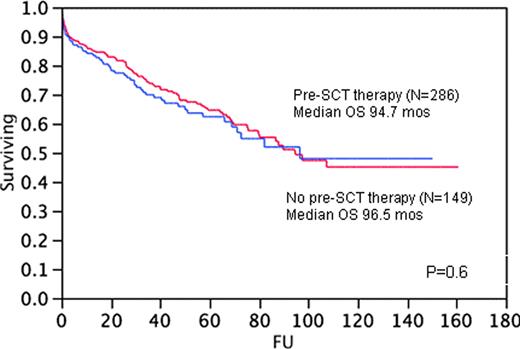
The median (range) age of pts at the time of SCT was 57.4 years (26-75); 260 (60%) were males. The median (range) duration from AL diagnosis to SCT was 4 mos (1-87), and 284 (65%) pts were alive at the time of analysis. Melphalan 200/m2 (271 pts) or Mel/TBI (17 pts) was used for conditioning in 66% pts, whereas the remaining one-third had reduced doses of melphalan (100-160 mg/m2). The median OS for pts that received pre-SCT therapy (N=286) compared with those who did not (N=149) was 94.7 and 96.5 months, respectively (P=0.6) (Fig 1). Among the group of pts who underwent pre-SCT therapy and were evaluable for response, the median OS for those with a hematologic response (N=42) and no hematologic response (N=42) was 82.1 and 51 months (P=0.2), respectively (Fig 2).
Our study demonstrates no difference in the OS of AL patients who received a pre-SCT therapy compared with those who received a SCT after their diagnosis of AL. In patients receiving pre-SCT therapy, there was a trend towards reduced OS among those with no hematologic response to pre-transplant therapy, but this difference was not significant.
Kumar:Celgene: Consultancy, Research Funding; Millennium: Research Funding; Merck: Consultancy, Research Funding; Novartis: Research Funding; Genzyme: Consultancy, Research Funding; Cephalon: Research Funding. Dispenzieri:Celgene: Honoraria, Research Funding; Binding Site: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal