Abstract
Abstract 3564
Autologous stem cell transplant (ASCT) with high-dose therapy (HDT) is an effective treatment modality for multiple myeloma (MM). MM is the leading indication for ASCT in North America. It was shown in a recent meta-analysis that a strategy of using ASCT early in the course of myeloma improves progression free survival (PFS) and quality of life. However, this study addressed only patients who had received conventional chemotherapy. Over the last decade, novel agents (thalidomide, bortezomib, and lenalidomide) have replaced conventional chemotherapy for induction in myeloma. These agents have demonstrated superior response rates when compared to conventional chemotherapy, and there is some indication that using novel therapies for induction before ASCT improves the duration of response and overall survival (OS). However, the question of whether early ASCT is the best strategy in the era of novel agents remains unanswered. We performed a retrospective analysis in order to compare the outcomes of patients with newly diagnosed MM treated with novel agents who received an early versus late ASCT.
179 newly diagnosed MM patients were treated or referred to The Ohio State University for ASCT between October 2006 and December 2009. All patients in the analysis received either thalidomide, bortezomib, or lenalidomide as part of their induction regimen and went on to receive HDT and ASCT. We compared the outcomes of patients who received ASCT within 12 months of diagnosis (early group, N=134) to those who received ASCT at a later date (late group, N=45). All patients received melphalan 140mg/m2 or 200mg/m2 as preparative regimen. Kaplan-Meier estimates were used to compare PFS and OS.
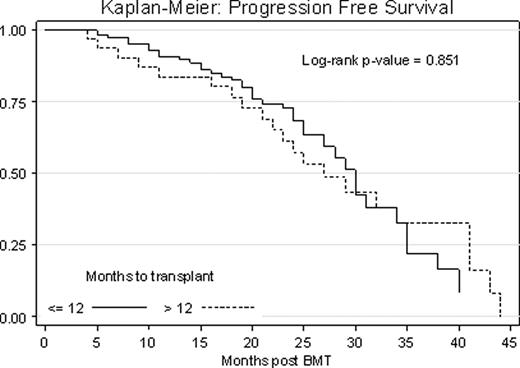
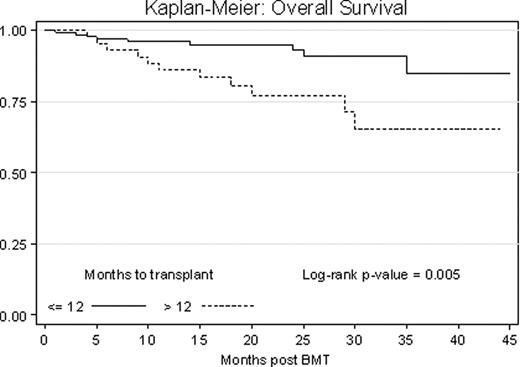
In our sample of 179 subjects there were no statistically significant differences in age, sex, race, performance status, comorbidity index score, disease stage at diagnosis, genetic risk and preparative regimen dose between the two groups. The median time from diagnosis to transplant was 7.2 months in the early group (N=134) and 17.7 months in the late group (N=45). In the early group, 81% of patients had received one line of treatment before transplant vs. 49% in the late group (p < 0.001). The overall response rate (ORR) prior to transplant was 90% (9% complete (CR), 31% very good (VGPR), 45% partial (PR)) in the early group, and 83% (9% CR, 22% VGPR, 47% PR) in the late group (p = 0.277). The ORR post transplant was 92% in the early group and 82% in the late group (p = 0.082), with a statistically significant proportion of patients in the early group obtaining CR (52% vs. 27%, p = 0.003). One year non-relapse mortality was 2% for both groups. At a median follow up of 22 months, 36% vs. 47% of patients had progressed (p = 0.29) and 7.5% vs. 24% had died due to disease progression (p = 0.005) in the early vs. late group. Median PFS was 30 months vs. 27 months with 1 year and 3 years PFS of 83% vs. 75% and 64% vs. 55% in the early vs. late group respectively (p = 0.851, Fig. 1). Of patients who had only one therapy pre transplant, results trended toward equivalent PFS in the two groups (p = 0.090), however more than 1 line of therapy and late transplant resulted in a progression hazard ratio of 2.43 (p = 0.050). OS was significantly better in the early group vs. the late group (p = 0.005, Fig. 2). On Cox regression analysis, a late ASCT resulted in a mortality hazard ratio of 3.30.
An early ASCT in newly diagnosed MM patients receiving novel agents for induction resulted in an improved OS but not PFS. Patients who are responding well to their first line treatment may have equivalent PFS regardless of time to ASCT. Patients who have had ≥ 2 lines of treatment and underwent ASCT within 12 months had significantly improved PFS compared to those who received ≥ 2 lines of treatment but were transplanted later. Therefore, patients who have not responded to their first line regimen and are within 12 months of diagnosis may have the greatest benefit from ASCT. An extended analysis will be presented at the meeting. A multicenter randomized study comparing early vs. late transplant is underway.
Phillips:NCI/NIH: Research Funding; NCCM Grant: Research Funding; ARRA RC2 Grant: Research Funding. Byrd:Genzyme Corporation: Research Funding.
This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal