Abstract
Abstract 3527
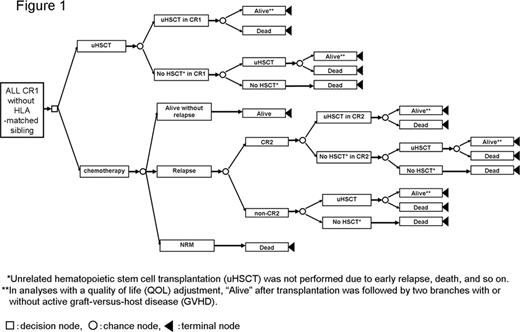
With modern intensive chemotherapy, about 90% of adult patients with Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL) achieve complete remission. However, the overall survival rate drops due to the high rate of relapse. Therefore, the establishment of optimal post-remission therapy is important, and the efficacy of allogeneic hematopoietic stem cell transplantation (HSCT) from a human leukocyte antigen (HLA)-matched sibling in first remission (CR1) has been demonstrated through clinical studies using genetic randomization. However, the efficacy of unrelated HSCT for adult patients with Ph-negative ALL in CR1 who lack an HLA-matched sibling remains unclear. Decision analysis is a statistical technique that aids the clinical decision-making process under uncertainty, especially in situations where a well-designed clinical trial is practically difficult to perform. We previously demonstrated through a decision analysis that HSCT is superior to chemotherapy (CTx) alone in CR1 for adult patients with Ph-negative ALL who have an HLA-matched sibling, even after an adjustment for quality of life (QOL) (Kako S et al, BMT 45 supp. p414, 2010). In a similar manner, we performed a decision analysis based on the decision tree (Figure 1) to evaluate the efficacy of unrelated HSCT for adult patients with Ph-negative ALL in CR1 who lack an HLA-matched sibling. The transition probabilities and utilities were estimated from studies of the Japan Adult Leukemia Study Group (JALSG) (ALL93; n=122, ALL97; n=119), the database of the Japan Marrow Donor Program (JMDP) (HSCT in CR1: n=231), and the literature. The primary outcome measure was the 10-year survival probability with or without a QOL adjustment, in which we especially consider the presence of chronic graft-versus-host disease (GVHD). Subgroup analyses were performed according to risk stratification based on the white blood cell count and cytogenetics, and according to age stratification with a cutoff of 35 years. In the whole population, the superiority of unrelated HSCT in CR1 was demonstrated in analyses both with and without a QOL adjustment (40.6% vs. 31.1% and 43.6% vs. 31.9%, respectively). A probabilistic sensitivity analysis using a Monte Carlo simulation supported these results. A similar tendency was observed in all subgroups (Table 1). The decision model was sensitive to the probability of disease-free survival following CTx and the probability of overall survival following HSCT in CR1 in standard-risk and higher-aged patients. In conclusion, to improve the probability of long-term survival, unrelated HSCT in CR1 is recommended for patients who lack an HLA-matched sibling donor. However, improvement of CTx in the future may change the result.
Expected 10-year survival probabilities with and without adjusting for quality of life (QOL)
| . | Expected survival probability without a QOL adjustment . | Expected survival probability With a QOL adjustment . | ||
|---|---|---|---|---|
| . | HSCT . | Chemotherapy . | HSCT . | Chemotherapy . |
| All patients | 43.6% | 31.8% | 40.6% | 31.0% |
| Standard-risk patients | 44.0% | 36.7% | 40.9% | 36.0% |
| High-risk patients | 43.7% | 23.8% | 40.6% | 23.1% |
| Lower-aged patients | 44.3% | 30.0% | 41.2% | 29.2% |
| Higher-aged patients | 42.1% | 33.1% | 39.1% | 32.6% |
| . | Expected survival probability without a QOL adjustment . | Expected survival probability With a QOL adjustment . | ||
|---|---|---|---|---|
| . | HSCT . | Chemotherapy . | HSCT . | Chemotherapy . |
| All patients | 43.6% | 31.8% | 40.6% | 31.0% |
| Standard-risk patients | 44.0% | 36.7% | 40.9% | 36.0% |
| High-risk patients | 43.7% | 23.8% | 40.6% | 23.1% |
| Lower-aged patients | 44.3% | 30.0% | 41.2% | 29.2% |
| Higher-aged patients | 42.1% | 33.1% | 39.1% | 32.6% |
Abbreviations: HSCT, hematopoietic stem cell transplantation.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal