Abstract
Abstract 3468
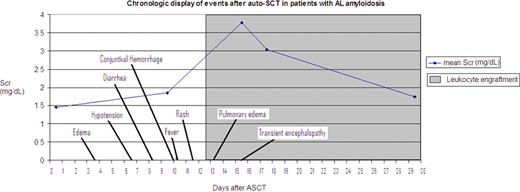
Engraftment syndrome (ES) is a complication of hematopoietic stem cell transplantation classically characterized by fever, rash, diarrhea, weight gain and non-cardiogenic pulmonary edema. Renal failure has been observed but is not considered to be a major part of the syndrome. We have noted that acute renal failure (ARF) is common around the time of leukocyte engraftment in light chain amyloidosis (AL) patients after autologous stem cell transplant (ASCT). We suspect this is due to ES and this retrospective review was conducted to further study this entity. Data were collected from 377 AL patients who underwent ASCT from 7/1997 to 10/2009. Patients who experienced an elevation of serum creatinine (Scr) > 0.5 mg/dl within 4 days of leukocyte engraftment were selected. Patients who carried a diagnosis of ES were also included regardless of Scr elevation. Nephrotoxic insults were reviewed. Vitals were collected. Forty-one patients met criteria. Four patients were found not to have ES, 2 sepsis, 1 allergic interstitial nephritis (AIN), 1 acute cellular rejection. ES was noted in 37 (9.8%) patients, 2 presented with mainly pulmonary ES and 8 had both pulmonary and renal involvement. The degree of renal failure varied from mild and self-limited to severe and requiring dialysis. The renal failure is usually preceded by fever, rash, increasing edema, relative hypotension and low urine sodium (Figure 1). Treatment with glucocorticoids (60 mg/d) was successful in reversing the renal failure in most patients but those with pulmonary involvement required higher doses. Our study showed that ES presents more commonly with renal failure than pulmonary involvement in AL patients. Further complications may be prevented by early treatment with corticosteroids once infection is ruled out. Dosing depends on concurrent pulmonary findings. Distinguishing ES with AIN is difficult as presentations are similar but AIN will continue until the offending drug is removed while ES generally responds quickly to steroids alone.
Dispenzieri:Celgene: Honoraria, Research Funding; Binding Site: Honoraria. Kumar:Celgene: Consultancy, Research Funding; Millennium: Research Funding; Merck: Consultancy, Research Funding; Novartis: Research Funding; Genzyme: Consultancy, Research Funding; Cephalon: Research Funding.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal