Abstract
Abstract 3414
We have previously shown that adherence to imatinib therapy is the single most important factor determining the degree of molecular response achieved by CML patients (Marin et al, JCO 2010). We now study the relation between adherence to imatinib and the probabilities of losing CCyR and of imatinib failure in patients receiving long term therapy.
We measured the adherence to imatinib in 87 consecutive chronic phase CML patients who had received imatinib 400 mg day as first line therapy for a median of 59.7 months before enrolment, all of whom were in complete cytogenetic response (CCyR). Adherence levels were monitored during a 3-month period using microelectronic monitoring devices (MEMS, see below) and patients were followed subsequently for a median of 15 months. Data from the patients were censored in March 2010, when our previous work was published, to ensure that the results of this study were not influenced by possible changes in a patient's behavior.
MEMS is an electronic device fitted into the cap of a normal looking medication bottle that automatically records each time the bottle is opened. MEMS are considered as the ‘gold standard' for measuring adherence. We also measured the imatinib plasma level, the presence of kinase domain mutations and the following factors assessed at diagnosis: demographic data, hemoglobin, leukocytes, Sokal risk group, BCR-ABL1 transcript type, hOCT1 transcript levels, and the presence of the 1236C>T polymorphism in ABCB1. The study protocol was approved by the Research Ethics Committee and patients gave written informed consent to participate.
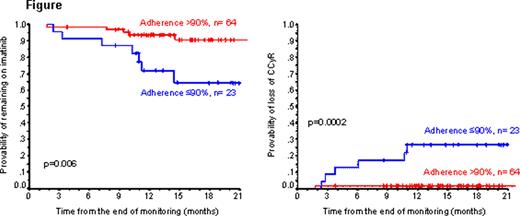
Adherence was defined as the quantity of imatinib actually taken divided by the amount prescribed expressed at a percentage. The median adherence was 97.6% range 24–100%). In 23 (26.4%) patients adherence was ≤90% (median 76%) and in 12 (13.8%) ≤80% (median 63%). During the follow up 7 (8%) patients lost their CCyR, and 12 (13.8%) discontinued the imatinib therapy (7 because of loss of CCyR, 2 because of failure to achieve MMR and 3 because of side effects). In multivariate analysis the adherence rate was the only independent predictor for loss of cytogenetic response (RR=0.95, p=0.0001) and discontinuation of imatinib therapy (RR= 0.97, p=0.009). When we categorized the adherence rate as (≤90% or >90%) we found that at 18 months the 23 patients with an adherence rate ≤90% had a higher probability of losing the CCyR (26.8% vs 1.5%, p=0.0002) and a lower probability of remaining on imatinib (64.5% vs 90.6%, p=0.006) than the 64 patients with an adherence rate >90% (Figure). In summary we have shown that poor adherence to imatinib is the principal factor contributing to the loss of cytogenetic responses and treatment failure in Patients on long term therapy.
Marin:Bristol-Myers Squibb: Consultancy; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal