Abstract
Abstract 338
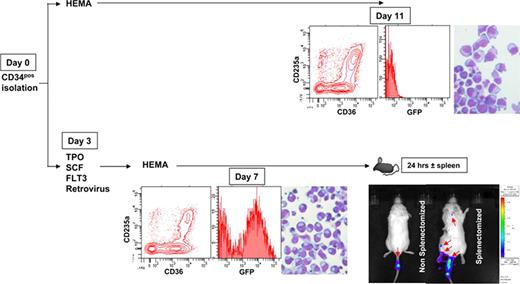
Ex vivo expanded erythroblasts (EBs) are red blood cell precursors with proliferative capacity that have the potential to serve as alternative transfusion product. In the present study, we investigated the biodistribution and persistence of human EBs expanded ex vivo from cord blood following intravenous administration to NOD/SCID/IL2Rγnull mice. In the first experiment, 107 EBs generated ex vivo from cord blood under Human Erythroid Massive Amplification (HEMA) culture conditions (Migliaccio G et al. Blood Cells Mol Dis. 2002;28:169) were labeled with CFSE and transfused via the tail vein into NOD/SCID/IL2Rγnull mice which had been bled (200 μL) 24 hrs earlier to increase erythropoietin (EPO) levels. The presence of human EBs in bone marrow (BM), spleen and blood of the transfused recipient mice was analyzed by flow cytometry for CSFE and human CD235a. At day 4, 1.5 – 5% of cells in BM and spleen of the animals were CD235apos but no human cells were detectable in blood. To clarify failure of human EBs to generate red blood cells in mice, a second cohort of mice was given 25×106 expanded EBs and sacrificed 4 days thereafter. Their tissues, including BM, liver and spleen, were examined by immunohistochemistry for expression of human markers. Human CD235apos cells were found in the spleen, representing up to 18% of total cells spleen cells of transfused recipients, but the cells were trapped inside larger CD235aneg cells, probably of murine origin. These results indicate that lodging of human EBs in the spleen, where they are probably destroyed by the macrophages, may represent a barrier to using mouse models as a surrogate assay for investigating transfused human EBs. To test this hypothesis, we analyzed the fate and biodistribution of human EBs into normal vs. splenectomized NOD/SCID/IL2Rγnull mice. Cell biodistribution was analyzed using bioluminescence imaging (BLI), following retroviral-mediated transfer of eGFP and the external Gaussia luciferase genes (Santos et al Nat Med 15: 338, 2009) into expanding cord blood-derived EBs. Cord blood-derived CD34pos cells were either expanded in HEMA culture (as control) or cultured for 3 days with TPO, SCF and FLT3L before retroviral transduction. After 3 days, the cells were cultured under HEMA conditions to induce EBs expansion. Mature EBs were detectable after 11 days of culture in the untransduced, control group and the cells expanded 67-fold. By contrast, transduced cord blood cells matured by day 5–7 and amplified only 24-fold (see Figure). Transduction efficiency, as reflected by GFP expression, was on the order of 32–50% in expanded EBs. All the recipient mice were bled 10 hrs before injection (200 μL). Half of them were splenectomized 24 hrs earlier. Mice were given 15×106 each expanded EBs together with 20 units of EPO and the cell biodistribution analyzed by imaging 24 hrs later (see Figure). In intact mice, BLI signal was virtually undetectable (other than in the tail). In contrast, in the splenectomized mice, significant signal levels were also observed in the body of the animals (see Figure). Four days after injection, mice were sacrificed and the presence of human CD235apos cells in the marrow, liver, spleen (intact animals only) and blood analyzed. CD235apos cells were detectable in the marrow (20%) and liver (6%) of the splenectomized mice while in intact animals they were mainly detected in spleen (15%). Detection of human red cells in blood, however, remained low in all the cases. Overall, we have established a model for the tracking and quantification of human EBs transfused into NOD/SCID/IL2Rγnull mice. This model will be very valuable to investigate the in vivo function and persistence of human EBs expanded under different conditions and thereby define the therapeutic potential of ex vivo generated human EBs derived from different stem cell sources.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal