Abstract
Abstract 2946
The achievement of complete remission (CR) is the crucial step for a long-lasting response and prolonged survival after autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM). The European Group for Blood and Marrow Transplantation (EBMT) criteria for CR include the negativity of serum and urine immunofixation (IFE) and less than 5% of bone marrow plasma cells (BMPCs). Additionally, the International Myeloma Working Group (IMWG) has even proposed a stringent CR category, which requires to rule out the clonal nature of the BMPCs. However, few studies have addressed this issue in patients with MM and negative IFE. The aim of the present study was to determine the impact of plasma cell count in the bone marrow aspirate on the long-term outcome of patients with MM with negative IFE after ASCT.
Thirty-five patients (16M/19F; median age at ASCT 55 years, range 26–68) with MM who underwent ASCT from March 1994 to December 2008, were studied. All patients had achieved a negative serum and urine IFE after high dose therapy with melphalan-based regimens. Bone marrow aspirate was performed when negative serum and urine IFE was achieved and at least three months from ASCT (median 3.24 months). The analysis was based on microscopic revision for May-Grünwald-Giemsa stained bone marrow smears performed according to standard procedures. BMPC percentage was calculated independently by two observers counting 500 bone marrow total nucleated cells in random areas from two different slides (1000 cells on each patient).
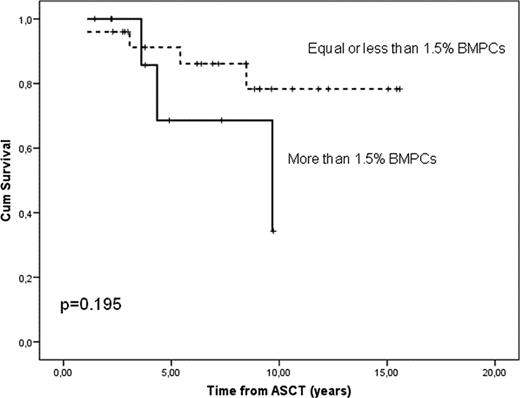
Median BMPCs percentage was 0.8 (range 0.1–5.8). Only two patients had more than 3% BPMCs. These results are in contrast with a recent report from the Mayo Clinic group, where 14% of the patients with MM and negative IFE had 5% or more BMPCs. In univariate Cox-model regression analysis, the number of BMPCs significantly correlated with progression-free survival (PFS)(p=0.021) with no impact on overall survival (OS)(p=0.92). This statistical significance on PFS was retained in the multivariate analysis, when baseline prognostic factors such as age, hemoglobin level, serum creatinine, β2-microglobulin and Durie-Salmon stage were added to the model (p=0.003). To establish the best predictive cut-off for progression and survival, a receptor-operator curve (ROC) analysis was developed. It showed the value of 1.5% BMPCs, with a sensitivity of 53%, specificity of 90% and area under the curve of 0.66 for predicting progression. Ten patients had more than 1.5% BMPC, and 25 equal or less than 1.5% BMPC. Median PFS was 8.5 years (CI 95% 2.6 to 14.3) and was not reached in patients with ≤1.5% BMPCs versus 3.1 years in patients with >1.5% BMPCs, with a hazard ratio probability to progression of 3.02 (CI 95% 1.18 to 9.71)(p=0.016) in the group with more than 1.5% of BMPCs (Figure 1). Median OS was not reached in patients with ≤1.5% compared with a median of 9.7 years in those with more than 1.5% BMPCs (p=0.195) (Figure 2). It is likely that serological CR with very low percentage of BMPCs (i.e. ≤1.5%) is equivalent to negative MRD assessed by MFC or molecular studies. In fact, all 8 patients in continued CR between 9 and 16 years beyond ASCT (“operational cures”) are in the group with ≤1.5% BMPCs, while all patients in the group with >1.5% BPMC have relapsed within the first 9 years from ASCT (Figure 1).
The percentage of BMPCs in patients with MM in CR after ASCT is a strong predictor of progression. Bone marrow morphology examination is an easy, inexpensive, and non-time consuming test and it should be the first step in the estimation of the residual tumor mass in patients with MM in CR after ASCT.
Progression-free survival (PFS) in patients with MM with negative immunofixation electrophoresis after ASCT, according to number of bone marrow plasma cells (BMPCs).
Progression-free survival (PFS) in patients with MM with negative immunofixation electrophoresis after ASCT, according to number of bone marrow plasma cells (BMPCs).
Overall survival (OS) in patients with MM with negative immunofixation electrophoresis after ASCT, according to number of bone marrow plasma cells (BMPCs).
Overall survival (OS) in patients with MM with negative immunofixation electrophoresis after ASCT, according to number of bone marrow plasma cells (BMPCs).
Rosiñol:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cibeira:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Blade:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal