Abstract
Abstract 2931
The incidence of sMDS is increasing due to improved survival of patients (pts) treated with chemotherapy (CT) or radiotherapy (RT) for other cancers. While studies have demonstrated hematologic improvement (HI) and survival benefits of AZA in pts with primary MDS (pMDS) (Lancet Oncol 2009;10:223), the effects of AZA in sMDS, considered rarer (5-10% of MDS diagnoses) (J Natl Cancer Inst 2008;100:1542) and more difficult to treat, are unknown. AVIDA, a longitudinal, US, multicenter, prospective registry of pts in community-based clinics receiving AZA, is the largest database of AZA-treated pts in the world and includes a large cohort of sMDS pts. We compared the tolerability of and response rates to AZA in sMDS vs pMDS pts in the AVIDA database.
MDS pt data were collected at registry entry (baseline), and then quarterly using electronic data capture, between October, 2006 and July, 2010. Treating physicians determined AZA dose, dosing schedule, and treatment duration. Baseline characteristics of sMDS and pMDS pts were evaluated but formal statistical tests comparing cohorts were intentionally not performed to avoid Type I errors. Rates of IWG-2000-defined HI or possibly better responses (HI+) were assessed centrally and compared between sMDS and pMDS cohorts (each assessment included only pts eligible for improvement). RBC and platelet transfusion independence (TI) were also evaluated between groups using logistic regression analyses with patients stratified by International Prognostic Scoring System (IPSS) scores (higher [score >1] vs lower [score ≤1]) and transfusion status at baseline, with age and months since diagnosis included as covariates. Odds ratios (sMDS to pMDS) and 95% confidence intervals (CI) were reported from these models.
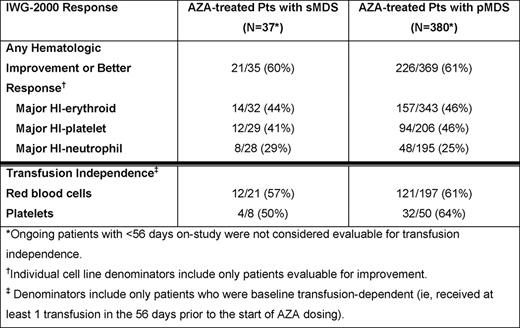
At data cut-off in July 2010, 37/417 pts (8.9%) in the registry had sMDS associated with exposure to RT, CT, or radioiodine (n=33), benzene (n=2), or radiation (n=2). Median times since diagnosis for pts with sMDS and pMDS were 1 month (range 0 – 69) and 3 months (0 – 207), and median ages were 71 years (range 41 – 86) and 75 years (29 – 91), respectively. At baseline, for pts with available IPSS scores, a larger proportion of pts with sMDS than pts with pMDS had IPSS higher-risk scores (55% vs 30%) and IPSS poor cytogenetics (59% vs 17%). Additionally, a higher proportion of sMDS vs pMDS pts had chromosome 7 abnormalities (47% vs 11%), 2–3 cytopenias (76% vs 62%), and infections requiring IV antibiotics (41% vs 16%); but similar proportions had >10% blasts (18% of both cohorts) and were dependent on RBC (57% vs 52%) and platelet (22% vs 13%) transfusions at baseline. Median follow-up was 5.9 months (range 0.2 – 24) in the sMDS and 6.7 months (0.1 – 37) in the pMDS cohorts, and median numbers of AZA treatment cycles were 4 (range 1 – 21) and 5 (1 – 26), respectively. In both the sMDS and pMDS groups, the most common treatment dose and schedules were 75 mg/m2 AZA (91% and 83%, respectively) for 5 consecutive days (46% and 55%) in ≤28-day cycles (45% and 54%). Pts with sMDS had a high rate of HI+, which was comparable to that in pts with pMDS (Table ). Rates of RBC TI in baseline RBC transfusion-dependent pts with sMDS vs pMDS were 57% vs 61%, and of platelet TI for baseline platelet transfusion-dependent sMDS vs pMDS pts were 50% vs 64% (Table ). Odds ratios from the logistic regression models were 1.4 (95%CI: 0.6, 3.5; p=0.47) and 0.6 (95%CI: 0.2, 1.4; p=0.23) for RBC TI and platelet TI, respectively, after adjusting for the other covariates in the model. Grade 3 or 4 adverse events were similar in the 2 groups, with the exception of higher frequencies of thrombocytopenia (27% vs 11%) and infections (24% vs. 12%) in sMDS vs pMDS pts, respectively.
Pts with sMDS treated with AZA had rates of HI or better responses comparable to those of pMDS patients, despite worse pretreatment disease characteristics. AZA was well tolerated by pts with sMDS and pMDS. A diagnosis of sMDS alone should not preclude treatment with the disease-modifying drug, azacitidine.
Sekeres:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Off Label Use: Azacitidine is approved in the US for treatment of patients with the FAB myelodysplastic syndrome (MDS) subtypes: Refractory anemia (RA) or refractory anemia with ringed sideroblasts (RARS) (if accompanied by neutropenia or thrombocytopenia or requiring transfusions), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEB-T), and chronic myelomonocytic leukemia (CMML); and is approved in the EU for IPSS Int-2 and High risk MDS, CMML with 10–29 percent marrow blasts without myeloproliferative disorder, and AML with 20–30% blasts and multi-lineage dysplasia, according to WHO classification. This abstract describes azacitidine use in secondary MDS. Komrokji:Celgene: Research Funding, Speakers Bureau. Maciejewski:Celgene: Research Funding; Eisai: Research Funding; Alexion: Consultancy. List:Celgene: Research Funding. Street:Celgene: Employment. Swern:Celgene Corporation: Employment. Sullivan:Celgene: Employment, Equity Ownership. Grinblatt:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal