Abstract
Abstract 293
The serine/threonine Pim kinases are known to play an important role in several signal transduction pathways, including those regulated by c-Myc, N-Myc, FLT3-ITD, BCR-ABL, HOXA9, and EWS fusions. Pim kinases are up regulated in some hematologic malignancies such as Acute Myeloid Leukemia (AML) and Chronic Lymphocytic Leukemia. Pim kinases were first identified as a proviral integration site in the mice that overexpress c-Myc and enhance lymphomagenesis. We have previously shown that SMI-4a, a novel benzylidene-thiazolidine-2,4-dione small molecule inhibitor of the Pim kinases inhibited the growth of precursor T-cell lymphoblastic leukemia in the mouse (Lin, YW. et al. Blood, 2010). We have also reported that SMI-4a in combination with a few MEK inhibitors or standard chemotherapeutic agents including daunorubicin, Ara-C, and 6-thioguanine synergistically killed myeloid and lymphoblastic leukemic cell lines as well as primary patient leukemic blasts (Lin, YW. et al. Blood, 2010, AACR 2010 abstract).
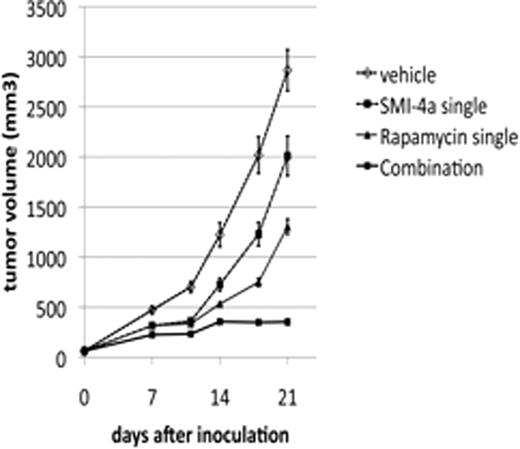
In the current study, we found that the combination of Rapamycin and Pim kinase inhibitors including SMI-4a, SMI-20a, SMI-24a, and K00135 synergistically killed MV4-11, a myeloid leukemia cell line harboring FLT3-ITD. We also show that a combination of SMI-4a and Rapamycin significantly inhibited subcutaneous tumor growth of MV4-11 expressing firefly-luciferase in NOD/SCID mice, which was determined using conventional caliper measurement and bioluminescent analysis in the region of interest. Although the combination of those drugs caused a loss of appetite in NOD/SCID mice in the first week of the treatment, the mice became tolerable and did not lose weight from the second week. In addition, none of those drugs alone or in combination caused any adverse affects in wild type FVB mice.
A combination of SMI-4a and Rapamycin also synergistically kills primary AML blasts including patients with or without expression of the FLT3-ITD. The treatment with SMI-4a or Rapamycin as a single agent down regulates phosphorylation of two substrates of the mTORC1 pathway, 4E-BP1 and S6K, while the combination significantly decreased their phosphorylation compared with single agent treatments, which is consistent with the synergistic effect. We found that SMI-4a increased phosphorylation of AMPK and decreased phosphorylation of mTOR at serine 2448 leading to down regulation of the mTORC1 pathway. In contrast, Rapamycin induced down regulation of 4E-BP1 and S6K without affecting phosphorylation of AMPK and mTOR. SMI-4a induced apoptotic cell death that was characterized by down regulation of MCL1, cleavage of Caspase 3, and nuclear condensation, whereas Rapamycin did not induce these changes. Together those two inhibitors modify different signaling pathways to synergistically kill AML blasts in vitro and in vivo.
Tholanikunnel:Vortex Biotechnology: Employment. Kraft:Vortex Biotechnology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal